Intensive pharmacological immunosuppression allows for repetitive liver gene transfer with recombinant adenovirus in nonhuman primates
- PMID: 20087317
- PMCID: PMC2862529
- DOI: 10.1038/mt.2009.312
Intensive pharmacological immunosuppression allows for repetitive liver gene transfer with recombinant adenovirus in nonhuman primates
Abstract
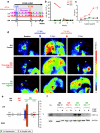
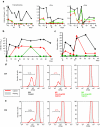
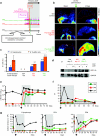
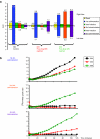
Repeated administration of gene therapies is hampered by host immunity toward vectors and transgenes. Attempts to circumvent antivector immunity include pharmacological immunosuppression or alternating different vectors and vector serotypes with the same transgene. Our studies show that B-cell depletion with anti-CD20 monoclonal antibody and concomitant T-cell inhibition with clinically available drugs permits repeated liver gene transfer to a limited number of nonhuman primates with recombinant adenovirus. Adenoviral vector-mediated transfer of the herpes simplex virus type 1 thymidine kinase (HSV1-tk) reporter gene was visualized in vivo with a semiquantitative transgene-specific positron emission tomography (PET) technique, liver immunohistochemistry, and immunoblot for the reporter transgene in needle biopsies. Neutralizing antibody and T cell-mediated responses toward the viral capsids were sequentially monitored and found to be repressed by the drug combinations tested. Repeated liver transfer of the HSV1-tk reporter gene with the same recombinant adenoviral vector was achieved in macaques undergoing a clinically feasible immunosuppressive treatment that ablated humoral and cellular immune responses. This strategy allows measurable gene retransfer to the liver as late as 15 months following the first adenoviral exposure in a macaque, which has undergone a total of four treatments with the same adenoviral vector.
Figures

Similar articles
-
Transient and intensive pharmacological immunosuppression fails to improve AAV-based liver gene transfer in non-human primates.J Transl Med. 2012 Jun 15;10:122. doi: 10.1186/1479-5876-10-122. J Transl Med. 2012. PMID: 22704060 Free PMC article.
-
Transient depletion of specific immune cell populations to improve adenovirus-mediated transgene expression in the liver.Liver Int. 2015 Apr;35(4):1274-89. doi: 10.1111/liv.12571. Epub 2014 May 10. Liver Int. 2015. PMID: 24754307
-
Inhibition of costimulation allows for repeated systemic administration of adenoviral vector in rhesus monkeys.Gene Ther. 2004 Feb;11(3):241-52. doi: 10.1038/sj.gt.3302152. Gene Ther. 2004. PMID: 14737083
-
Circumventing antivector immunity: potential use of nonhuman adenoviral vectors.Hum Gene Ther. 2014 Apr;25(4):285-300. doi: 10.1089/hum.2013.228. Epub 2014 Mar 25. Hum Gene Ther. 2014. PMID: 24499174 Free PMC article. Review.
-
Immune response to helper dependent adenoviral mediated liver gene therapy: challenges and prospects.Curr Gene Ther. 2007 Oct;7(5):297-305. doi: 10.2174/156652307782151452. Curr Gene Ther. 2007. PMID: 17979676 Review.
Cited by
-
Lentiviral gene therapy prevents anti-human acid α-glucosidase antibody formation in murine Pompe disease.Mol Ther Methods Clin Dev. 2022 May 4;25:520-532. doi: 10.1016/j.omtm.2022.04.016. eCollection 2022 Jun 9. Mol Ther Methods Clin Dev. 2022. PMID: 35662813 Free PMC article.
-
Ultrasound-assisted nonviral gene transfer of AQP1 to the irradiated minipig parotid gland restores fluid secretion.Gene Ther. 2015 Sep;22(9):739-49. doi: 10.1038/gt.2015.36. Epub 2015 Apr 14. Gene Ther. 2015. PMID: 25871828 Free PMC article.
-
IGF2-tagging of GAA promotes full correction of murine Pompe disease at a clinically relevant dosage of lentiviral gene therapy.Mol Ther Methods Clin Dev. 2022 Sep 24;27:109-130. doi: 10.1016/j.omtm.2022.09.010. eCollection 2022 Dec 8. Mol Ther Methods Clin Dev. 2022. PMID: 36284764 Free PMC article.
-
Helper-dependent adenovirus achieve more efficient and persistent liver transgene expression in non-human primates under immunosuppression.Gene Ther. 2015 Nov;22(11):856-65. doi: 10.1038/gt.2015.64. Epub 2015 Jul 23. Gene Ther. 2015. PMID: 26125605
-
Evaluation of monocytes as carriers for armed oncolytic adenoviruses in murine and Syrian hamster models of cancer.Hum Gene Ther. 2012 Dec;23(12):1258-68. doi: 10.1089/hum.2012.043. Epub 2012 Oct 26. Hum Gene Ther. 2012. PMID: 22985305 Free PMC article.
References
-
- Bessis N, GarciaCozar FJ., and , Boissier MC. Immune responses to gene therapy vectors: influence on vector function and effector mechanisms. Gene Ther. 2004;11 Suppl 1:S10–S17. - PubMed
-
- Mingozzi F., and , High KA. Immune responses to AAV in clinical trials. Curr Gene Ther. 2007;7:316–324. - PubMed
-
- Smith TA, White BD, Gardner JM, Kaleko M., and , McClelland A. Transient immunosuppression permits successful repetitive intravenous administration of an adenovirus vector. Gene Ther. 1996;3:496–502. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

