Enhancing the therapeutic effect against ovarian cancer through a combination of viral oncolysis and antigen-specific immunotherapy
- PMID: 20087318
- PMCID: PMC2862517
- DOI: 10.1038/mt.2009.318
Enhancing the therapeutic effect against ovarian cancer through a combination of viral oncolysis and antigen-specific immunotherapy
Abstract
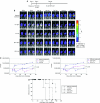
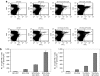
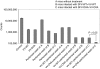
Cancer therapy using oncolytic viruses represents a promising new approach for controlling ovarian cancer. In this study, we have circumvented the limitation of repeated vaccination by employing different virus vectors, Semliki Forest Virus (SFV) and vaccinia virus (VV) for boosting the immune response. We found that infection of tumor-bearing mice with VV followed by infection with SFV or vice versa leads to enhanced antitumor effects against murine ovarian surface epithelial carcinoma (MOSEC) tumors. Furthermore, infection with VV-ovalbumin (OVA) followed by infection with SFV-OVA or vice versa was found to lead to enhanced OVA-specific CD8(+) T-cell immune responses. In addition, we found that infection with SFV-OVA followed by infection with VV-OVA leads to enhanced antitumor effects in vivo and enhanced tumor killing in vitro through a combination of viral oncolysis and antigen-specific immunity. The clinical implications of this study are discussed.
Figures



References
-
- Greenlee RT, Murray T, Bolden S., and , Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7–33. - PubMed
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. - PubMed
-
- Baum M, Ebb S., and , Brooks M. Biological fall out from trials of adjuvant tamoxifen in early ovarian cancer. In: Salmon SE (ed.). Adjuvant therapy of cancer V1. Saunders: Philadelphia, pp 269–274; 1990.
-
- Schwartz PE. Current diagnosis and treatment modalities for ovarian cancer. Cancer Treat Res. 2002;107:99–118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

