Erk signaling and chromatin remodeling in MMTV promoter activation by progestins
- PMID: 20087429
- PMCID: PMC2807634
- DOI: 10.1621/nrs.07008
Erk signaling and chromatin remodeling in MMTV promoter activation by progestins
Abstract
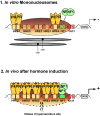
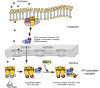
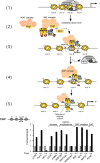
Transcription from the mouse mammary tumor virus (MMTV) promoter can be induced by progestins. The progesterone receptor (PR) binds to a cluster of five hormone responsive elements (HREs) and activates the promoter by synergistic interactions with the ubiquitous transcription factor, nuclear factor 1 (NF1). Progesterone treatment of cells in culture leads to activation of the Src/Ras/Erk/Msk1 cascade. Selective inhibition of Erk, or its target kinase Msk1, interferes with chromatin remodeling and blocks MMTV activation. A complex of activated PR, Erk and Msk1 is recruited to promoter after 5 min of hormone treatment and phosphorylates histone H3 at serine 10. This modification promotes the displacement of HP1gamma and subsequent chromatin remodeling. Progestin treatment leads to the recruitment of the BAF complex, which selectively displaces histones H2A and H2B from the nucleosome containing the HREs. The acetyltransferase PCAF is also required for induction of progesterone target genes and acetylates histone H3 at K14, an epigenetic mark, which interacts with Brg1 and Brm, anchoring the BAF complex to chromatin. In nucleosomes assembled on either MMTV or mouse rDNA promoter sequences, SWI/SNF displaces histones H2A and H2B from MMTV, but not from the rDNA nucleosome. Thus, the outcome of nucleosome remodeling by purified SWI/SNF depends on DNA sequence. The resultant H3/H4 tetramer particle is then the substrate for subsequent events in induction. Thus, initial activation of the MMTV promoter requires activation of several kinases and PCAF leading to phosphoacetylation of H3, and recruitment of BAF with subsequent removal of H2A/H2B.
Figures
Similar articles
-
Chromatin remodeling and control of cell proliferation by progestins via cross talk of progesterone receptor with the estrogen receptors and kinase signaling pathways.Ann N Y Acad Sci. 2006 Nov;1089:59-72. doi: 10.1196/annals.1386.025. Ann N Y Acad Sci. 2006. PMID: 17261755 Review.
-
Minireview: role of kinases and chromatin remodeling in progesterone signaling to chromatin.Mol Endocrinol. 2010 Nov;24(11):2088-98. doi: 10.1210/me.2010-0027. Epub 2010 May 19. Mol Endocrinol. 2010. PMID: 20484412 Free PMC article. Review.
-
Induction of progesterone target genes requires activation of Erk and Msk kinases and phosphorylation of histone H3.Mol Cell. 2006 Nov 3;24(3):367-81. doi: 10.1016/j.molcel.2006.10.011. Mol Cell. 2006. PMID: 17081988
-
Complex role of histone H1 in transactivation of MMTV promoter chromatin by progesterone receptor.J Steroid Biochem Mol Biol. 2002 Dec;83(1-5):15-23. doi: 10.1016/s0960-0760(02)00253-4. J Steroid Biochem Mol Biol. 2002. PMID: 12650697
-
DNA instructed displacement of histones H2A and H2B at an inducible promoter.Mol Cell. 2004 Nov 5;16(3):439-52. doi: 10.1016/j.molcel.2004.10.025. Mol Cell. 2004. PMID: 15525516
Cited by
-
Global signalling network analysis of luminal T47D breast cancer cells in response to progesterone.Front Endocrinol (Lausanne). 2022 Aug 11;13:888802. doi: 10.3389/fendo.2022.888802. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36034422 Free PMC article.
-
Elongating under Stress.Genet Res Int. 2011;2011:326286. doi: 10.4061/2011/326286. Epub 2011 Sep 7. Genet Res Int. 2011. PMID: 22567351 Free PMC article.
-
PPARγ recruitment to active ERK during memory consolidation is required for Alzheimer's disease-related cognitive enhancement.J Neurosci. 2014 Mar 12;34(11):4054-63. doi: 10.1523/JNEUROSCI.4024-13.2014. J Neurosci. 2014. PMID: 24623782 Free PMC article.
-
The p38 SAPK is recruited to chromatin via its interaction with transcription factors.J Biol Chem. 2010 Oct 8;285(41):31819-28. doi: 10.1074/jbc.M110.155846. Epub 2010 Aug 3. J Biol Chem. 2010. PMID: 20682780 Free PMC article.
-
New role for granulocyte colony-stimulating factor-induced extracellular signal-regulated kinase 1/2 in histone modification and retinoic acid receptor α recruitment to gene promoters: relevance to acute promyelocytic leukemia cell differentiation.Mol Cell Biol. 2011 Apr;31(7):1409-18. doi: 10.1128/MCB.00756-10. Epub 2011 Jan 24. Mol Cell Biol. 2011. PMID: 21262770 Free PMC article.
References
-
- Ballare C., Uhrig M., Bechtold T., Sancho E., Di Domenico M., Migliaccio A., Auricchio F., Beato M. Two domains of the progesterone receptor interact with the estrogen receptor and are required for progesterone activation of the c-Src/Erk pathway in mammalian cells. Mol Cell Biol. 2003;23:1994–2008. - PMC - PubMed
-
- Beato M., Herrlich P., Schutz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

