Control of oocyte release by progesterone receptor-regulated gene expression
- PMID: 20087433
- PMCID: PMC2807638
- DOI: 10.1621/nrs.07012
Control of oocyte release by progesterone receptor-regulated gene expression
Abstract
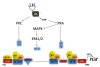
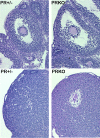
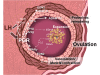
The progesterone receptor (PGR) is a nuclear receptor transcription factor that is essential for female fertility, in part due to its control of oocyte release from the ovary, or ovulation. In all mammals studied to date, ovarian expression of PGR is restricted primarily to granulosa cells of follicles destined to ovulate. Granulosa cell expression of PGR is induced by the pituitary Luteinizing Hormone (LH) surge via mechanisms that are not entirely understood, but which involve activation of Protein Kinase A and modification of Sp1/Sp3 transcription factors on the PGR promoter. Null mutations for PGR or treatment with PGR antagonists block ovulation in all species analyzed, including humans. The cellular mechanisms by which PGR regulates ovulation are currently under investigation, with several downstream pathways having been identified as PGR-regulated and potentially involved in follicular rupture. Interestingly, none of these PGR-regulated genes has been demonstrated to be a direct transcriptional target of PGR. Rather, in ovarian granulosa cells, PGR may act as an inducible coregulator for constitutively bound Sp1/Sp3 transcription factors, which are key regulators for a discrete cohort of ovulatory genes.
Figures


Similar articles
-
A novel pathway involving progesterone receptor, endothelin-2, and endothelin receptor B controls ovulation in mice.Mol Endocrinol. 2006 Nov;20(11):2784-95. doi: 10.1210/me.2006-0093. Epub 2006 Aug 3. Mol Endocrinol. 2006. PMID: 16887885
-
The critical roles of progesterone receptor (PGR) in ovulation, oocyte developmental competence and oviductal transport in mammalian reproduction.Reprod Domest Anim. 2012 Aug;47 Suppl 4:288-96. doi: 10.1111/j.1439-0531.2012.02088.x. Reprod Domest Anim. 2012. PMID: 22827383 Review.
-
Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases.Proc Natl Acad Sci U S A. 2000 Apr 25;97(9):4689-94. doi: 10.1073/pnas.080073497. Proc Natl Acad Sci U S A. 2000. PMID: 10781075 Free PMC article.
-
Transactivation of the progesterone receptor gene in granulosa cells: evidence that Sp1/Sp3 binding sites in the proximal promoter play a key role in luteinizing hormone inducibility.Mol Endocrinol. 2003 Mar;17(3):436-49. doi: 10.1210/me.2002-0252. Epub 2002 Dec 12. Mol Endocrinol. 2003. PMID: 12554796
-
Control of ovulation in mice by progesterone receptor-regulated gene networks.Mol Hum Reprod. 2009 Dec;15(12):821-8. doi: 10.1093/molehr/gap082. Epub 2009 Oct 8. Mol Hum Reprod. 2009. PMID: 19815644 Free PMC article. Review.
Cited by
-
2,3,5,4'-Tetrahydroxystilbene-2-O-β-D-Glucoside improves female ovarian aging.Front Cell Dev Biol. 2022 Aug 30;10:862045. doi: 10.3389/fcell.2022.862045. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36111333 Free PMC article.
-
Differential Expression of DUB Genes in Ovarian Cells Treated with Di-2-Ethylhexyl Phthalate.Int J Mol Sci. 2020 Mar 4;21(5):1755. doi: 10.3390/ijms21051755. Int J Mol Sci. 2020. PMID: 32143396 Free PMC article.
-
Nuclear progestin receptor (pgr) knockouts in zebrafish demonstrate role for pgr in ovulation but not in rapid non-genomic steroid mediated meiosis resumption.Front Endocrinol (Lausanne). 2015 Mar 19;6:37. doi: 10.3389/fendo.2015.00037. eCollection 2015. Front Endocrinol (Lausanne). 2015. PMID: 25852646 Free PMC article.
-
Steroidogenic Factor 1 Regulation of the Hypothalamic-Pituitary-Ovarian Axis of Adult Female Mice.Endocrinology. 2022 Apr 1;163(4):bqac028. doi: 10.1210/endocr/bqac028. Endocrinology. 2022. PMID: 35247045 Free PMC article.
-
BMPR2 is required for postimplantation uterine function and pregnancy maintenance.J Clin Invest. 2013 Jun;123(6):2539-50. doi: 10.1172/JCI65710. Epub 2013 May 8. J Clin Invest. 2013. PMID: 23676498 Free PMC article.
References
-
- Ashkenazi H., Cao X., Motola S., Popliker M., Conti M., Tsafriri A. Epidermal growth factor family members: endogenous mediators of the ovulatory response. Endocrinology. 2005;146:77–84. - PubMed
-
- Attardi B. J., Burgenson J., Hild S. A., Reel J. R., Blye R. P. CDB-4124 and its putative monodemethylated metabolite, CDB-4453, are potent antiprogestins with reduced antiglucocorticoid activity: in vitro comparison to mifepristone and CDB-2914. Mol Cell Endocrinol. 2002;188:111–23. - PubMed
-
- Attardi B. J., Burgenson J., Hild S. A., Reel J. R. In vitro antiprogestational/antiglucocorticoid activity and progestin and glucocorticoid receptor binding of the putative metabolites and synthetic derivatives of CDB-2914, CDB-4124, and mifepristone. J Steroid Biochem Mol Biol. 2004;88:277–88. - PubMed
-
- Butler T. A., Woessner J. F., Jr. Gelatinases and endogenous inhibitors in the preovulatory rat ovary. Ann N Y Acad Sci. 1994;732:444–6. - PubMed
-
- Butler T. A., Zhu C., Mueller R. A., Fuller G. C., Lemaire W. J., Woessner J. F., Jr. Inhibition of ovulation in the perfused rat ovary by the synthetic collagenase inhibitor SC 44463. Biol Reprod. 1991;44:1183–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

