Methylation of dietary flavones increases their metabolic stability and chemopreventive effects
- PMID: 20087474
- PMCID: PMC2808020
- DOI: 10.3390/ijms10115002
Methylation of dietary flavones increases their metabolic stability and chemopreventive effects
Abstract
Dietary flavones have promising chemoprotective properties, in particular with regard to cancer, but problems with low oral bioavailability and sometimes unacceptable toxicity have made their use as protective additives to normal diets questionable. However, methylation of free phenolic hydroxyl groups leads to derivatives not susceptible to glucuronic acid or sulfate conjugation, resulting in increased metabolic stability. Methylation also leads to greatly improved transport through biological membranes, such as in intestinal absorption, and much increased oral bioavailability. Recent studies also indicate that methylation results in derivatives with increasing potency to kill cancer cells. They also show high potency towards inhibition of hormone-regulating enzymes, e.g., aromatase, important in the causation of breast cancer. Methylation of the flavones may also result in derivatives with diminished toxic side-effects and improved aqueous solubility. In conclusion, it appears that methylation of dietary flavones as well as of other food products may produce derivatives with much improved health effects.
Keywords: cancer prevention; flavonoids; methoxyflavones; methylation.
Figures



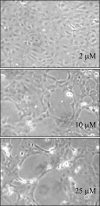

Similar articles
-
Methylation of dietary flavones greatly improves their hepatic metabolic stability and intestinal absorption.Mol Pharm. 2007 Nov-Dec;4(6):826-32. doi: 10.1021/mp700071d. Epub 2007 Oct 25. Mol Pharm. 2007. PMID: 17958394 Review.
-
Methoxylated flavones, a superior cancer chemopreventive flavonoid subclass?Semin Cancer Biol. 2007 Oct;17(5):354-62. doi: 10.1016/j.semcancer.2007.05.002. Epub 2007 May 13. Semin Cancer Biol. 2007. PMID: 17574860 Free PMC article. Review.
-
Methylated flavonoids have greatly improved intestinal absorption and metabolic stability.Drug Metab Dispos. 2006 Oct;34(10):1786-92. doi: 10.1124/dmd.106.011122. Epub 2006 Jul 25. Drug Metab Dispos. 2006. PMID: 16868069
-
Cancer chemopreventive properties of orally bioavailable flavonoids--methylated versus unmethylated flavones.Biochem Pharmacol. 2007 May 1;73(9):1288-96. doi: 10.1016/j.bcp.2006.12.028. Epub 2006 Dec 28. Biochem Pharmacol. 2007. PMID: 17250812 Free PMC article.
-
Aromatase inhibition by bioavailable methylated flavones.J Steroid Biochem Mol Biol. 2007 Oct;107(1-2):127-9. doi: 10.1016/j.jsbmb.2007.01.006. Epub 2007 Jun 6. J Steroid Biochem Mol Biol. 2007. PMID: 17624765 Free PMC article.
Cited by
-
3'-8″- Biflavones: A Review of Their Structural Diversity, Natural Occurrence, Role in Plants, Extraction and Identification.Molecules. 2024 Sep 29;29(19):4634. doi: 10.3390/molecules29194634. Molecules. 2024. PMID: 39407564 Free PMC article. Review.
-
Anticancer effects of Bilberry anthocyanins compared with NutraNanoSphere encapsulated Bilberry anthocyanins.Mol Clin Oncol. 2018 Feb;8(2):330-335. doi: 10.3892/mco.2017.1520. Epub 2017 Nov 29. Mol Clin Oncol. 2018. PMID: 29399357 Free PMC article.
-
Production and Anti-Melanoma Activity of Methoxyisoflavones from the Biotransformation of Genistein by Two Recombinant Escherichia coli Strains.Molecules. 2017 Jan 4;22(1):87. doi: 10.3390/molecules22010087. Molecules. 2017. PMID: 28054996 Free PMC article.
-
Evaluation of the effects of hesperidin on fresh and frozen-thawed semen quality using two different cryopreservation methods in Simmental bull.Anim Reprod. 2022 Oct 11;19(3):e20220042. doi: 10.1590/1984-3143-AR2022-0042. eCollection 2022. Anim Reprod. 2022. PMID: 36313596 Free PMC article.
-
2, 3-Dihydro-3β-methoxy Withaferin-A Lacks Anti-Metastasis Potency: Bioinformatics and Experimental Evidences.Sci Rep. 2019 Nov 22;9(1):17344. doi: 10.1038/s41598-019-53568-6. Sci Rep. 2019. PMID: 31757995 Free PMC article.
References
-
- Middleton EJ, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000;52:673–751. - PubMed
-
- Williams RJ, Spencer JPE, Rice-Evans C. Flavonoids: Antioxidants and signalling molecules? Free Radic. Biol. Med. 2004;36:838–849. - PubMed
-
- Doostdar H, Burke MD, Mayer RT. Bioflavonoids: Selective substrates and inhibitors for cytochrome P450 CYP1A and CYP1B1. Toxicology. 2000;144:31–38. - PubMed
-
- Guengerich FP, Chun Y-J, Kim D, Gillam EMJ, Shimada T.Cytochrome P450 1B1: A target for inhibition in anticarcinogenesis strategies Mut Res 2003523–524., 173–182. - PubMed
-
- Wen X, Walle T. Preferential induction of CYP1B1 by benzo[a]pyrene in human oral epithelial cells: Impact on DNA adduct formation and prevention by polyphenols. Carcinogenesis. 2005;26:1774–1781. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

