The disease-causing mutations in the carboxyl terminus of the cone cyclic nucleotide-gated channel CNGA3 subunit alter the local secondary structure and interfere with the channel active conformational change
- PMID: 20088482
- PMCID: PMC2829136
- DOI: 10.1021/bi901960u
The disease-causing mutations in the carboxyl terminus of the cone cyclic nucleotide-gated channel CNGA3 subunit alter the local secondary structure and interfere with the channel active conformational change
Abstract
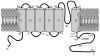
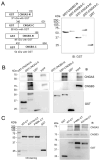
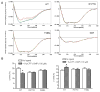
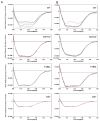
The cone photoreceptor cyclic nucleotide-gated (CNG) channel plays a pivotal role in phototransducton. Mutations in the channel subunits are associated with achromatopsia and progressive cone dystrophy in humans. More than 50 mutations have been identified in the channel CNGA3 subunit, with 50% of them located in the carboxyl (C) terminus. This study investigates the defects of the two frequently occurring mutations, R377W and F488L, in the C-terminus of CNGA3. Ratiometric measurement of the intracellular Ca(2+) concentration and electrophysiological recordings showed the loss of functional activity of the mutant channels in an HEK293 heterologous expression system. Immunofluorescence labeling revealed an apparent cytosolic aggregation of the mutant channels compared to the wild type (WT). The R377W and F488L mutants, expressed and purified from Escherichia coli as glutathione S-transferase (GST) fused to the CNGA3 C-terminal domain, showed no negative effects on interactions with the channel subunits. Circular dichroism spectrum analyses were performed to examine the structural impact of the mutations. Although the R377W and F488L C-termini mutants retained stable, folded structures, the secondary structures of both mutants differed from the WT protein. Furthermore, the WT C-terminus exhibited a significant decrease in alpha-helical content in response to the channel ligands, while this allosteric transition was diminished in the two mutants. This is the first study showing the structural impact of the disease-causing mutations in the cone CNG channel subunit. The observed alterations in the local secondary structure and active conformational change may confer an adverse effect on the channel's activity and cellular processing.
Figures




References
-
- Zagotta WN, Siegelbaum SA. Structure and function of cyclic nucleotide-gated channels. Annu Rev Neurosci. 1996;19:235–263. - PubMed
-
- Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels. Physiol Rev. 2002;82:769–824. - PubMed
-
- Weitz D, Ficek N, Kremmer E, Bauer PJ, Kaupp UB. Subunit stoichiometry of the CNG channel of rod photoreceptors. Neuron. 2002;36:881–889. - PubMed
-
- Peng C, Rich ED, Varnum MD. Subunit configuration of heteromeric cone cyclic nucleotide-gated channels. Neuron. 2004;42:401–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

