Nonpeptidic tetrafluorophenoxymethyl ketone cruzain inhibitors as promising new leads for Chagas disease chemotherapy
- PMID: 20088534
- PMCID: PMC2838180
- DOI: 10.1021/jm901633v
Nonpeptidic tetrafluorophenoxymethyl ketone cruzain inhibitors as promising new leads for Chagas disease chemotherapy
Abstract
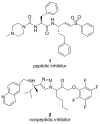
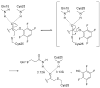
A century after discovering that the Trypanosoma cruzi parasite is the etiological agent of Chagas disease, treatment is still plagued by limited efficacy, toxicity, and the emergence of drug resistance. The development of inhibitors of the major T. cruzi cysteine protease, cruzain, has been demonstrated to be a promising drug discovery avenue for this neglected disease. Here we establish that a nonpeptidic tetrafluorophenoxymethyl ketone cruzain inhibitor substantially ameliorates symptoms of acute Chagas disease in a mouse model with no apparent toxicity. A high-resolution crystal structure confirmed the mode of inhibition and revealed key binding interactions of this novel inhibitor class. Subsequent structure-guided optimization then resulted in inhibitor analogues with improvements in potency despite minimal or no additions in molecular weight. Evaluation of the analogues in cell culture showed enhanced activity. These results suggest that nonpeptidic tetrafluorophenoxymethyl ketone cruzain inhibitors have the potential to fulfill the urgent need for improved Chagas disease chemotherapy.
Figures



Similar articles
-
Tetrafluorophenoxymethyl ketone cruzain inhibitors with improved pharmacokinetic properties as therapeutic leads for Chagas' disease.Bioorg Med Chem Lett. 2015 Nov 1;25(21):4834-4837. doi: 10.1016/j.bmcl.2015.06.066. Epub 2015 Jun 23. Bioorg Med Chem Lett. 2015. PMID: 26144347 Free PMC article.
-
Identification of a new class of nonpeptidic inhibitors of cruzain.J Am Chem Soc. 2008 May 21;130(20):6404-10. doi: 10.1021/ja710254m. Epub 2008 Apr 25. J Am Chem Soc. 2008. PMID: 18435536 Free PMC article.
-
Synthesis, biological evaluation, and structure-activity relationships of potent noncovalent and nonpeptidic cruzain inhibitors as anti-Trypanosoma cruzi agents.J Med Chem. 2014 Mar 27;57(6):2380-92. doi: 10.1021/jm401709b. Epub 2014 Mar 3. J Med Chem. 2014. PMID: 24533839
-
Examination of multiple Trypanosoma cruzi targets in a new drug discovery approach for Chagas disease.Bioorg Med Chem. 2022 Mar 15;58:116577. doi: 10.1016/j.bmc.2021.116577. Epub 2022 Feb 1. Bioorg Med Chem. 2022. PMID: 35189560 Review.
-
Cruzain inhibitors: efforts made, current leads and a structural outlook of new hits.Drug Discov Today. 2015 Jul;20(7):890-8. doi: 10.1016/j.drudis.2015.02.004. Epub 2015 Feb 16. Drug Discov Today. 2015. PMID: 25697479 Review.
Cited by
-
New approaches for dissecting protease functions to improve probe development and drug discovery.Nat Struct Mol Biol. 2012 Jan 5;19(1):9-16. doi: 10.1038/nsmb.2203. Nat Struct Mol Biol. 2012. PMID: 22218294 Free PMC article. Review.
-
Advances in Chagas disease drug development: 2009-2010.Curr Opin Infect Dis. 2010 Dec;23(6):609-16. doi: 10.1097/QCO.0b013e3283402956. Curr Opin Infect Dis. 2010. PMID: 20885320 Free PMC article. Review.
-
Structure-Aided Computational Design of Triazole-Based Targeted Covalent Inhibitors of Cruzipain.Molecules. 2024 Sep 5;29(17):4224. doi: 10.3390/molecules29174224. Molecules. 2024. PMID: 39275072 Free PMC article.
-
Cruzain structures: apocruzain and cruzain bound to S-methyl thiomethanesulfonate and implications for drug design.Acta Crystallogr F Struct Biol Commun. 2019 Jun 1;75(Pt 6):419-427. doi: 10.1107/S2053230X19006320. Epub 2019 May 13. Acta Crystallogr F Struct Biol Commun. 2019. PMID: 31204688 Free PMC article.
-
Integrated Computational Approaches for Drug Design Targeting Cruzipain.Int J Mol Sci. 2024 Mar 27;25(7):3747. doi: 10.3390/ijms25073747. Int J Mol Sci. 2024. PMID: 38612558 Free PMC article.
References
-
- Dias JC. Elimination of Chagas disease transmission: perspectives. Mem Inst Oswaldo Cruz. 2009;104 (Suppl I):41–45. - PubMed
-
- de Souza W. Chagas’ disease: facts and reality. Microbes and Infection. 2007;9:544–545. - PubMed
-
- Castro JA, deMecca MM, Bartel LC. Toxic side effects of drugs used to treat Chagas’ disease (American Trypanosomiasis) Human Exp Toxicol. 2006;25:471–479. - PubMed
-
- Filardi LS, Brener Z. Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used clinically in Chagas’ disease. Trans R Soc Trop Med Hyg. 1987;81:755–759. - PubMed
-
- Rodrigo D, Moreira M, Leite ACL, Ribeiro dos Santos R, Soares MBP. Approaches for the development of new anti-Trypanosoma cruzi agents. Curr Drug Targets. 2009;10:212–231. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical

