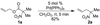Rapid decarboxylative allylation of nitroalkanes
- PMID: 20088536
- PMCID: PMC2821453
- DOI: 10.1021/ol902828p
Rapid decarboxylative allylation of nitroalkanes
Abstract
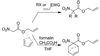
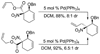
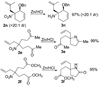
Allyl nitroacetates undergo decarboxylative allylation to provide tertiary nitroalkanes in high yield. Moreover, the transformations are complete within several minutes under ambient conditions. High yields result because O-allylation of the intermediate nitronates, which is typically problematic, is reversible under conditions of the decarboxylative allylation process. Lastly, the preparation of substrate allyl nitroacetates by tandem Knoevenagel/Diels-Alder sequences allows the facile synthesis of relatively complex substrates that undergo diastereoselective decarboxylative allylation.
Figures
Similar articles
-
Palladium-catalyzed regio-, diastereo-, and enantioselective allylation of nitroalkanes with monosubstituted allylic substrates.J Org Chem. 2013 Jul 5;78(13):6503-9. doi: 10.1021/jo400663d. Epub 2013 Jun 24. J Org Chem. 2013. PMID: 23741970
-
Enantioselective conjugate addition of nitroalkanes to vinyl sulfone: an organocatalytic access to chiral amines.Org Lett. 2009 Apr 16;11(8):1721-4. doi: 10.1021/ol9003349. Org Lett. 2009. PMID: 19317479
-
Synthesis of Chiral β-Amino Nitroalkanes via Rhodium-Catalyzed Asymmetric Hydrogenation.Org Lett. 2016 Jan 4;18(1):40-3. doi: 10.1021/acs.orglett.5b03158. Epub 2015 Dec 11. Org Lett. 2016. PMID: 26652759
-
Conjugate additions of nitroalkanes to electron-poor alkenes: recent results.Chem Rev. 2005 Mar;105(3):933-71. doi: 10.1021/cr040602r. Chem Rev. 2005. PMID: 15755081 Review. No abstract available.
-
A Regio- and Stereodivergent Synthesis of Homoallylic Amines by a One-Pot Cooperative-Catalysis-Based Allylic Alkylation/Hofmann Rearrangement Strategy.Angew Chem Int Ed Engl. 2019 Jul 29;58(31):10521-10527. doi: 10.1002/anie.201905426. Epub 2019 Jun 28. Angew Chem Int Ed Engl. 2019. PMID: 31132203 Free PMC article. Review.
Cited by
-
Deacylative allylation of nitroalkanes: unsymmetric bisallylation by a three-component coupling.Angew Chem Int Ed Engl. 2011 Feb 11;50(7):1688-91. doi: 10.1002/anie.201006273. Epub 2010 Dec 29. Angew Chem Int Ed Engl. 2011. PMID: 21308933 Free PMC article. No abstract available.
-
Trifluoromethylation of Secondary Nitroalkanes.Org Lett. 2017 Jun 2;19(11):2957-2960. doi: 10.1021/acs.orglett.7b01196. Epub 2017 May 23. Org Lett. 2017. PMID: 28535057 Free PMC article.
-
Deacylative allylation: allylic alkylation via retro-Claisen activation.J Am Chem Soc. 2011 Sep 21;133(37):14785-94. doi: 10.1021/ja205717f. Epub 2011 Aug 24. J Am Chem Soc. 2011. PMID: 21830777 Free PMC article.
-
Allylation of C-, N-, and O-Nucleophiles via a Mechanochemically-Driven Tsuji-Trost Reaction Suitable for Late-Stage Modification of Bioactive Molecules.Angew Chem Weinheim Bergstr Ger. 2024 Jan 2;136(1):e202314637. doi: 10.1002/ange.202314637. Epub 2023 Nov 29. Angew Chem Weinheim Bergstr Ger. 2024. PMID: 38516646 Free PMC article.
-
Migratory decarboxylative coupling of coumarins: synthetic and mechanistic aspects.Angew Chem Int Ed Engl. 2011 May 23;50(22):5157-61. doi: 10.1002/anie.201100765. Epub 2011 Apr 19. Angew Chem Int Ed Engl. 2011. PMID: 21506221 Free PMC article. No abstract available.
References
-
-
For reviews of allylation of aldimines see:Ding H, Friestad GK. Synthesis. 2005:2815–2829.Shibasaki M, Kanai M. Chem. Rev. 2008;108:2853–2873.
-
-
- Burger EC, Tunge JA. J. Am. Chem. Soc. 2006;128:10002–10003. - PubMed
-
- Yeagley AA, Chruma JJ. Org. Lett. 2007;9:2979–2882. - PubMed
-
- Ono N. The Nitro Group in Organic Synthesis. New York, NY: Feuer, H.; Wiley-VCH; 2001. pp. 140–147.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources