Inactivation of Lactobacillus leichmannii ribonucleotide reductase by 2',2'-difluoro-2'-deoxycytidine 5'-triphosphate: covalent modification
- PMID: 20088569
- PMCID: PMC2855214
- DOI: 10.1021/bi902132u
Inactivation of Lactobacillus leichmannii ribonucleotide reductase by 2',2'-difluoro-2'-deoxycytidine 5'-triphosphate: covalent modification
Abstract
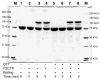
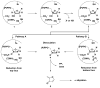
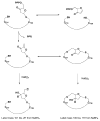
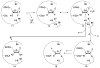
Ribonucleotide reductase (RNR) from Lactobacillus leichmannii, a 76 kDa monomer using adenosylcobalamin (AdoCbl) as a cofactor, catalyzes the conversion of nucleoside triphosphates to deoxynucleotides and is rapidly (<30 s) inactivated by 1 equiv of 2',2'-difluoro-2'-deoxycytidine 5'-triphosphate (F(2)CTP). [1'-(3)H]- and [5-(3)H]F(2)CTP were synthesized and used independently to inactivate RNR. Sephadex G-50 chromatography of the inactivation mixture revealed that 0.47 equiv of a sugar was covalently bound to RNR and that 0.71 equiv of cytosine was released. Alternatively, analysis of the inactivated RNR by SDS-PAGE without boiling resulted in 33% of RNR migrating as a 110 kDa protein. Inactivation of RNR with a mixture of [1'-(3)H]F(2)CTP and [1'-(2)H]F(2)CTP followed by reduction with NaBH(4), alkylation with iodoacetamide, trypsin digestion, and HPLC separation of the resulting peptides allowed isolation and identification by MALDI-TOF mass spectrometry (MS) of a (3)H/(2)H-labeled peptide containing C(731) and C(736) from the C-terminus of RNR accounting for 10% of the labeled protein. The MS analysis also revealed that the two cysteines were cross-linked to a furanone species derived from the sugar of F(2)CTP. Incubation of [1'-(3)H]F(2)CTP with C119S-RNR resulted in 0.3 equiv of sugar being covalently bound to the protein, and incubation with NaBH(4) subsequent to inactivation resulted in trapping of 2'-fluoro-2'-deoxycytidine. These studies and the ones in the preceding paper (DOI: 10.1021/bi9021318 ) allow proposal of a mechanism of inactivation of RNR by F(2)CTP involving multiple reaction pathways. The proposed mechanisms share many common features with F(2)CDP inactivation of the class I RNRs.
Figures






Similar articles
-
Inactivation of Lactobacillus leichmannii ribonucleotide reductase by 2',2'-difluoro-2'-deoxycytidine 5'-triphosphate: adenosylcobalamin destruction and formation of a nucleotide-based radical.Biochemistry. 2010 Feb 23;49(7):1396-403. doi: 10.1021/bi9021318. Biochemistry. 2010. PMID: 20088568 Free PMC article.
-
Mechanism of inactivation of human ribonucleotide reductase with p53R2 by gemcitabine 5'-diphosphate.Biochemistry. 2009 Dec 15;48(49):11612-21. doi: 10.1021/bi901588z. Biochemistry. 2009. PMID: 19899807 Free PMC article.
-
Insight into the mechanism of inactivation of ribonucleotide reductase by gemcitabine 5'-diphosphate in the presence or absence of reductant.Biochemistry. 2009 Dec 15;48(49):11622-9. doi: 10.1021/bi901590q. Biochemistry. 2009. PMID: 19899770 Free PMC article.
-
The function of adenosylcobalamin in the mechanism of ribonucleoside triphosphate reductase from Lactobacillus leichmannii.Curr Opin Chem Biol. 1998 Oct;2(5):650-5. doi: 10.1016/s1367-5931(98)80097-5. Curr Opin Chem Biol. 1998. PMID: 9818192 Review.
-
Development of ribonucleotide reductase inhibitors: a review on structure activity relationships.Mini Rev Med Chem. 2013 Nov;13(13):1862-72. doi: 10.2174/13895575113136660090. Mini Rev Med Chem. 2013. PMID: 24032510 Review.
Cited by
-
Molybdopterin biosynthesis-Mechanistic studies on a novel MoaA catalyzed insertion of a purine carbon into the ribose of GTP.Biochim Biophys Acta. 2015 Sep;1854(9):1073-7. doi: 10.1016/j.bbapap.2015.04.008. Epub 2015 Apr 17. Biochim Biophys Acta. 2015. PMID: 25896388 Free PMC article. Review.
-
Inactivation of Lactobacillus leichmannii ribonucleotide reductase by 2',2'-difluoro-2'-deoxycytidine 5'-triphosphate: adenosylcobalamin destruction and formation of a nucleotide-based radical.Biochemistry. 2010 Feb 23;49(7):1396-403. doi: 10.1021/bi9021318. Biochemistry. 2010. PMID: 20088568 Free PMC article.
-
One-electron oxidation of gemcitabine and analogs: mechanism of formation of C3' and C2' sugar radicals.J Am Chem Soc. 2014 Nov 5;136(44):15646-53. doi: 10.1021/ja5083156. Epub 2014 Oct 23. J Am Chem Soc. 2014. PMID: 25296262 Free PMC article.
-
Genetic characterization and role in virulence of the ribonucleotide reductases of Streptococcus sanguinis.J Biol Chem. 2014 Feb 28;289(9):6273-87. doi: 10.1074/jbc.M113.533620. Epub 2013 Dec 31. J Biol Chem. 2014. PMID: 24381171 Free PMC article.
-
Mechanism of inactivation of human ribonucleotide reductase with p53R2 by gemcitabine 5'-diphosphate.Biochemistry. 2009 Dec 15;48(49):11612-21. doi: 10.1021/bi901588z. Biochemistry. 2009. PMID: 19899807 Free PMC article.
References
-
- Plunkett W, Huang P, Gandhi V. Gemcitabine: actions and interactions. Nucleosides Nucleotides. 1997;16:1261–1270.
-
- Plunkett W, Huang P, Xu Y-Z, Heinemann V, Grunewald R, Gandhi V. Gemcitabine: metabolism, mechanisms of action, and self-potentiation. Sem Oncol. 1995;2:3–10. - PubMed
-
- Rivera F, López-Tarruella S, Vega-Villegas ME, Salcedo M. Treatment of advanced pancreatic cancer: from gemcitabine single agent to combinations and targeted therapy. Cancer Treat Rev. 2009;35:335–339. - PubMed
-
- Danesi R, Altavilla G, Giovannetti E, Rosell R. Pharmacogenetics of gemcitabine in non-small-cell lung cancer and other solid tumors. Pharmacogenetics. 2009;10:69–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

