Role of mitochondrial DNA damage in the development of diabetic retinopathy, and the metabolic memory phenomenon associated with its progression
- PMID: 20088705
- PMCID: PMC2935337
- DOI: 10.1089/ars.2009.2932
Role of mitochondrial DNA damage in the development of diabetic retinopathy, and the metabolic memory phenomenon associated with its progression
Abstract
Diabetic retinopathy does not halt after hyperglycemia is terminated; the retina continues to experience increased oxidative stress, suggesting a memory phenomenon. Mitochondrial DNA (mtDNA) is highly sensitive to oxidative damage. The goal is to investigate the role of mtDNA damage in the development of diabetic retinopathy, and in the metabolic memory. mtDNA damage and its functional consequences on electron transport chain (ETC) were analyzed in the retina from streptozotocin-diabetic rats maintained in poor control (PC, glycated hemoglobin >11%) for 12 months or PC for 6 months followed by good control (GC, GHb < 6.5%) for 6 months. Diabetes damaged retinal mtDNA and elevated DNA repair enzymes (glycosylase). ETC proteins that were encoded by the mitochondrial genome and the glycosylases were compromised in the mitochondria. Re-institution of GC after 6 months of PC failed to protect mtDNA damage, and ETC proteins remained subnormal. Thus, mtDNA continues to be damaged even after PC is terminated. Although the retina tries to overcome mtDNA damage by inducing glycosylase, they remain deficient in the mitochondria with a compromised ETC system. The process is further exacerbated by subsequent increased mtDNA damage providing no relief to the retina from a continuous cycle of damage, and termination of hyperglycemia fails to arrest the progression of retinopathy.
Figures
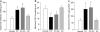
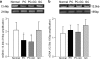
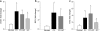



References
-
- Ayala–Torres S. Chen Y. Svoboda T. Rosenblatt J. Van Houten B. Analysis of gene-specific DNA damage and repair using quantitative polymerase chain reaction. Methods. 2000;22:135–147. - PubMed
-
- Ballinger SW. Bouder TG. Davis GS. Judice SA. Nicklas JA. Albertini RJ. Mitochondrial genome damage associated with cigarette smoking. Cancer Res. 1996;56:5692–5697. - PubMed
-
- Ballinger SW. Van Houtens B. Jin GF. Conklin CA. Godley BF. Hydrogen peroxide causes significant mitochondrial DNA damage in human RPE cells. Exp Eye Res. 1999;68:765–772. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

