RNA interference-based therapeutics for human immunodeficiency virus HIV-1 treatment: synthetic siRNA or vector-based shRNA?
- PMID: 20088715
- PMCID: PMC3745298
- DOI: 10.1517/14712590903448158
RNA interference-based therapeutics for human immunodeficiency virus HIV-1 treatment: synthetic siRNA or vector-based shRNA?
Abstract
Importance of the field: Despite the clinical benefits of highly active antiretroviral therapy (HAART), the prospect of life-long antiretroviral treatment poses significant problems, which has spurred interest in developing new drugs and strategies to treat HIV infection and eliminate persistent viral reservoirs. RNAi has emerged as a therapeutic possibility for HIV.
Areas covered in this review: We discuss progress in overcoming hurdles to translating transient and stable RNAi enabling technologies to clinical application for HIV; covering the past 2 - 3 years.
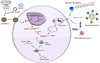
What the reader will gain: HIV inhibition can be achieved by transfection of chemically or enzymatically synthesized siRNAs or by DNA-based vector systems expressing short hairpin RNAs (shRNAs) that are processed intracellularly into siRNA. We compare these approaches, focusing on technical and safety issues that will guide the choice of strategy for clinical use.
Take home message: Introduction of synthetic siRNA into cells or its stable endogenous production using vector-driven shRNA have been shown to suppress HIV replication in vitro and, in some instances, in vivo. Each method has advantages and limitations in terms of ease of delivery, duration of silencing, emergence of escape mutants and potential toxicity. Both appear to have potential as future therapeutics for HIV, once the technical and safety issues of each approach are overcome.
Figures

References
-
- Scherer L, Rossi JJ, Weinberg MS. Progress and prospects: RNA-based therapies for treatment of HIV infection. Gene Ther. 2007 Jul;14(14):1057–1064. - PubMed
-
- Anderson J, Li MJ, Palmer B, Remling L, Li S, Yam P, et al. Safety and efficacy of a lentiviral vector containing three anti-HIV genes--CCR5 ribozyme, tat-rev siRNA, and TAR decoy--in SCID-hu mouse-derived T cells. Mol Ther. 2007 Jun;15(6):1182–1188. - PubMed
-
- Shankar P, Manjunath N, Lieberman J. The prospect of silencing disease using RNA interference. JAMA. 2005 Mar 16;293(11):1367–1373. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
