The mechanical properties of single fibrin fibers
- PMID: 20088938
- PMCID: PMC3010862
- DOI: 10.1111/j.1538-7836.2010.03745.x
The mechanical properties of single fibrin fibers
Abstract
Summary background: Blood clots perform the mechanical task of stemming the flow of blood.
Objectives: To advance understanding and realistic modeling of blood clot behavior we determined the mechanical properties of the major structural component of blood clots, fibrin fibers.
Methods: We used a combined atomic force microscopy (AFM)/fluorescence microscopy technique to determine key mechanical properties of single crosslinked and uncrosslinked fibrin fibers.
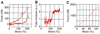
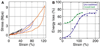
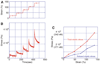
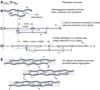
Results and conclusions: Overall, full crosslinking renders fibers less extensible, stiffer, and less elastic than their uncrosslinked counterparts. All fibers showed stress relaxation behavior (time-dependent weakening) with a fast and a slow relaxation time, 2 and 52 s. In detail, crosslinked and uncrosslinked fibrin fibers can be stretched to 2.5 and 3.3 times their original length before rupturing. Crosslinking increased the stiffness of fibers by a factor of 2, as the total elastic modulus, E(0), increased from 3.9 to 8.0 MPa and the relaxed, elastic modulus, E(infinity), increased from 1.9 to 4.0 MPa upon crosslinking. Moreover, fibers stiffened with increasing strain (strain hardening), as E(0) increased by a factor of 1.9 (crosslinked) and 3.0 (uncrosslinked) at strains epsilon > 110%. At low strains, the portion of dissipated energy per stretch cycle was small (< 10%) for uncrosslinked fibers, but significant (approximately 40%) for crosslinked fibers. At strains > 100%, all fiber types dissipated about 70% of the input energy. We propose a molecular model to explain our data. Our single fiber data can now also be used to construct a realistic, mechanical model of a fibrin network.
Conflict of interest statement
The authors state that they have no conflict of interest.
Figures

Comment in
-
Biomechanics in hemostasis and thrombosis.J Thromb Haemost. 2010 May;8(5):1027-9. doi: 10.1111/j.1538-7836.2010.03808.x. Epub 2010 Feb 11. J Thromb Haemost. 2010. PMID: 20149079
-
Short by one mechanism: a rebuttal.J Thromb Haemost. 2010 Sep;8(9):2089-90; author reply 2090-1. doi: 10.1111/j.1538-7836.2010.03952.x. J Thromb Haemost. 2010. PMID: 20553381 No abstract available.
Similar articles
-
Strength, deformability and toughness of uncrosslinked fibrin fibers from theoretical reconstruction of stress-strain curves.Acta Biomater. 2021 Dec;136:327-342. doi: 10.1016/j.actbio.2021.09.050. Epub 2021 Oct 2. Acta Biomater. 2021. PMID: 34606991 Free PMC article.
-
Evidence that αC region is origin of low modulus, high extensibility, and strain stiffening in fibrin fibers.Biophys J. 2010 Nov 3;99(9):3038-47. doi: 10.1016/j.bpj.2010.08.060. Biophys J. 2010. PMID: 21044602 Free PMC article.
-
Fibrin Fiber Stiffness Is Strongly Affected by Fiber Diameter, but Not by Fibrinogen Glycation.Biophys J. 2016 Mar 29;110(6):1400-10. doi: 10.1016/j.bpj.2016.02.021. Biophys J. 2016. PMID: 27028649 Free PMC article.
-
A comparison of the mechanical and structural properties of fibrin fibers with other protein fibers.Cell Biochem Biophys. 2007;49(3):165-81. doi: 10.1007/s12013-007-9001-4. Epub 2007 Oct 2. Cell Biochem Biophys. 2007. PMID: 17952642 Free PMC article. Review.
-
The molecular origins of the mechanical properties of fibrin.Biophys Chem. 2010 Nov;152(1-3):15-20. doi: 10.1016/j.bpc.2010.08.009. Biophys Chem. 2010. PMID: 20888119 Free PMC article. Review.
Cited by
-
Structural basis for the nonlinear mechanics of fibrin networks under compression.Biomaterials. 2014 Aug;35(25):6739-49. doi: 10.1016/j.biomaterials.2014.04.056. Epub 2014 May 16. Biomaterials. 2014. PMID: 24840618 Free PMC article.
-
To What Extent Are the Terminal Stages of Sepsis, Septic Shock, Systemic Inflammatory Response Syndrome, and Multiple Organ Dysfunction Syndrome Actually Driven by a Prion/Amyloid Form of Fibrin?Semin Thromb Hemost. 2018 Apr;44(3):224-238. doi: 10.1055/s-0037-1604108. Epub 2017 Aug 4. Semin Thromb Hemost. 2018. PMID: 28778104 Free PMC article. Review.
-
Fibrin Formation, Structure and Properties.Subcell Biochem. 2017;82:405-456. doi: 10.1007/978-3-319-49674-0_13. Subcell Biochem. 2017. PMID: 28101869 Free PMC article. Review.
-
Strength and failure of fibrin fiber branchpoints.J Thromb Haemost. 2010 May;8(5):1135-8. doi: 10.1111/j.1538-7836.2010.03824.x. Epub 2010 Feb 24. J Thromb Haemost. 2010. PMID: 20180818 Free PMC article.
-
Using molecular mechanics to predict bulk material properties of fibronectin fibers.PLoS Comput Biol. 2012;8(12):e1002845. doi: 10.1371/journal.pcbi.1002845. Epub 2012 Dec 27. PLoS Comput Biol. 2012. PMID: 23300425 Free PMC article.
References
-
- Weisel JW. The mechanical properties of fibrin for basic scientists and clinicians. Biophys Chem. 2004;112:267–276. - PubMed
-
- Lorand L. Acquired inhibitors of fibrin stabilization: a class of hemorrhagic disorders of diverse origins. In: Green D, editor. Anticoagulants, Physiologic, Pathologic and Pharmacologic. Boca Raton: CRC Press; 1994. pp. 169–191.
-
- Roberts WW, Lorand LL, Mockros LF. Viscoelastic properties of fibrin clots. Biorheology. 1973;10:29–42. - PubMed
-
- Benkherourou M, Gumery PY, Tranqui P. Quantification and macroscopic modeling of the nonlinear viscoelastic behavior of strained gels with varying fibrin concentrations. IEEE Trans Biomed Eng. 2000;47:1465–1475. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

