E. coli secreted protein F promotes EPEC invasion of intestinal epithelial cells via an SNX9-dependent mechanism
- PMID: 20088948
- PMCID: PMC2896985
- DOI: 10.1111/j.1462-5822.2010.01440.x
E. coli secreted protein F promotes EPEC invasion of intestinal epithelial cells via an SNX9-dependent mechanism
Abstract
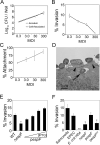
Enteropathogenic Escherichia coli (EPEC) infection requires the injection of effector proteins into intestinal epithelial cells (IECs) via type 3 secretion. Type 3-secreted effectors can interfere with IEC signalling pathways via specific protein-protein interactions. For example, E. coli secreted protein F (EspF) binds sorting nexin 9 (SNX9), an endocytic regulator, resulting in tubulation of the plasma membrane. Our aim was to determine the mechanism of EspF/SNX9-induced membrane tubulation. Point mutation of the SNX9 lipid binding domains or truncation of the EspF SNX9 binding domains significantly inhibited tubulation, as did inhibition of clathrin coated pit (CCP) assembly. Although characterized as non-invasive, EPEC are known to invade IECs in vitro and in vivo. Indeed, we found significant invasion of Caco-2 cells by EPEC, which, like tubulation, was blocked by pharmacological inhibition of CCPs. Interestingly, however, inhibition of dynamin activity did not prevent tubulation or EPEC invasion, which is in contrast to Salmonella invasion, which requires dynamin activity. Our data also indicate that EPEC invasion is dependent on EspF and its interaction with SNX9. Together, these findings suggest that EspF promotes EPEC invasion of IECs by harnessing the membrane-deforming activity of SNX9.
Figures






Similar articles
-
Clever Cooperation: Interactions Between EspF and Host Proteins.Front Microbiol. 2018 Nov 22;9:2831. doi: 10.3389/fmicb.2018.02831. eCollection 2018. Front Microbiol. 2018. PMID: 30524410 Free PMC article. Review.
-
EPEC effector EspF promotes Crumbs3 endocytosis and disrupts epithelial cell polarity.Cell Microbiol. 2017 Nov;19(11):10.1111/cmi.12757. doi: 10.1111/cmi.12757. Epub 2017 Jul 27. Cell Microbiol. 2017. PMID: 28618099 Free PMC article.
-
Tight junctions and enteropathogenic E. coli.Ann N Y Acad Sci. 2009 May;1165:169-74. doi: 10.1111/j.1749-6632.2009.04060.x. Ann N Y Acad Sci. 2009. PMID: 19538303 Free PMC article.
-
EspF of enteropathogenic Escherichia coli binds sorting nexin 9.J Bacteriol. 2006 Apr;188(8):3110-5. doi: 10.1128/JB.188.8.3110-3115.2006. J Bacteriol. 2006. PMID: 16585770 Free PMC article.
-
SNX9 - a prelude to vesicle release.J Cell Sci. 2009 Jan 1;122(Pt 1):5-11. doi: 10.1242/jcs.037135. J Cell Sci. 2009. PMID: 19092055 Review.
Cited by
-
Clever Cooperation: Interactions Between EspF and Host Proteins.Front Microbiol. 2018 Nov 22;9:2831. doi: 10.3389/fmicb.2018.02831. eCollection 2018. Front Microbiol. 2018. PMID: 30524410 Free PMC article. Review.
-
Escherichia albertii, a novel human enteropathogen, colonizes rat enterocytes and translocates to extra-intestinal sites.PLoS One. 2017 Feb 8;12(2):e0171385. doi: 10.1371/journal.pone.0171385. eCollection 2017. PLoS One. 2017. PMID: 28178312 Free PMC article.
-
Actin cytoskeleton manipulation by effector proteins secreted by diarrheagenic Escherichia coli pathotypes.Biomed Res Int. 2013;2013:374395. doi: 10.1155/2013/374395. Epub 2012 Dec 30. Biomed Res Int. 2013. PMID: 23509714 Free PMC article. Review.
-
EspF is crucial for Citrobacter rodentium-induced tight junction disruption and lethality in immunocompromised animals.PLoS Pathog. 2019 Jun 28;15(6):e1007898. doi: 10.1371/journal.ppat.1007898. eCollection 2019 Jun. PLoS Pathog. 2019. PMID: 31251784 Free PMC article.
-
Safeguarding intestine cells against enteropathogenic Escherichia coli by intracellular protein reaction, a preventive antibacterial mechanism.Proc Natl Acad Sci U S A. 2020 Mar 10;117(10):5260-5268. doi: 10.1073/pnas.1914567117. Epub 2020 Feb 24. Proc Natl Acad Sci U S A. 2020. PMID: 32094196 Free PMC article.
References
-
- Allen-Vercoe E, Waddell B, Livingstone S, Deans J, DeVinney R. Enteropathogenic Escherichia coli Tir translocation and pedestal formation requires membrane cholesterol in the absence of bundle-forming pili. Cell Microbiol. 2006;8:613–624. - PubMed
-
- Alto NM, Shao F, Lazar CS, Brost RL, Chua G, Mattoo S, et al. Identification of a bacterial type III effector family with G protein mimicry functions. Cell. 2006;124:133–145. - PubMed
-
- Andrade JRC, Da Viega VF, De Santa Rosa MR, Suassuna I. An endoctyic process in HEp-2 cells induced by enteropathogenic Escherichia coli. J Med Microbiol. 1989;28:49–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

