MUC16 provides immune protection by inhibiting synapse formation between NK and ovarian tumor cells
- PMID: 20089172
- PMCID: PMC2818693
- DOI: 10.1186/1476-4598-9-11
MUC16 provides immune protection by inhibiting synapse formation between NK and ovarian tumor cells
Abstract
Background: Cancer cells utilize a variety of mechanisms to evade immune detection and attack. Effective immune detection largely relies on the formation of an immune synapse which requires close contact between immune cells and their targets. Here, we show that MUC16, a heavily glycosylated 3-5 million Da mucin expressed on the surface of ovarian tumor cells, inhibits the formation of immune synapses between NK cells and ovarian tumor targets. Our results indicate that MUC16-mediated inhibition of immune synapse formation is an effective mechanism employed by ovarian tumors to evade immune recognition.
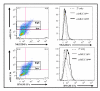
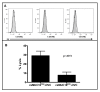
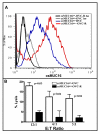
Results: Expression of low levels of MUC16 strongly correlated with an increased number of conjugates and activating immune synapses between ovarian tumor cells and primary naïve NK cells. MUC16-knockdown ovarian tumor cells were more susceptible to lysis by primary NK cells than MUC16 expressing controls. This increased lysis was not due to differences in the expression levels of the ligands for the activating receptors DNAM-1 and NKG2D. The NK cell leukemia cell line (NKL), which does not express KIRs but are positive for DNAM-1 and NKG2D, also conjugated and lysed MUC16-knockdown cells more efficiently than MUC16 expressing controls. Tumor cells that survived the NKL challenge expressed higher levels of MUC16 indicating selective lysis of MUC16(low) targets. The higher csMUC16 levels on the NKL resistant tumor cells correlated with more protection from lysis as compared to target cells that were never exposed to the effectors.
Conclusion: MUC16, a carrier of the tumor marker CA125, has previously been shown to facilitate ovarian tumor metastasis and inhibits NK cell mediated lysis of tumor targets. Our data now demonstrates that MUC16 expressing ovarian cancer cells are protected from recognition by NK cells. The immune protection provided by MUC16 may lead to selective survival of ovarian cancer cells that are more efficient in metastasizing within the peritoneal cavity and also at overcoming anti-tumor innate immune responses.
Figures






Similar articles
-
MUC16 suppresses human and murine innate immune responses.Gynecol Oncol. 2019 Mar;152(3):618-628. doi: 10.1016/j.ygyno.2018.12.023. Epub 2019 Jan 6. Gynecol Oncol. 2019. PMID: 30626487 Free PMC article.
-
Identification of Siglec-9 as the receptor for MUC16 on human NK cells, B cells, and monocytes.Mol Cancer. 2010 May 24;9:118. doi: 10.1186/1476-4598-9-118. Mol Cancer. 2010. PMID: 20497550 Free PMC article.
-
Boosting Natural Killer Cell-Mediated Targeting of Sarcoma Through DNAM-1 and NKG2D.Front Immunol. 2020 Jan 28;11:40. doi: 10.3389/fimmu.2020.00040. eCollection 2020. Front Immunol. 2020. PMID: 32082316 Free PMC article.
-
MUC16 (CA125): tumor biomarker to cancer therapy, a work in progress.Mol Cancer. 2014 May 29;13:129. doi: 10.1186/1476-4598-13-129. Mol Cancer. 2014. PMID: 24886523 Free PMC article. Review.
-
NK cell recognition and killing of melanoma cells is controlled by multiple activating receptor-ligand interactions.J Innate Immun. 2011;3(4):365-73. doi: 10.1159/000328505. Epub 2011 May 11. J Innate Immun. 2011. PMID: 21576932 Review.
Cited by
-
Targeted Mass Spectrometry of Longitudinal Patient Sera Reveals LTBP1 as a Potential Surveillance Biomarker for High-Grade Serous Ovarian Carcinoma.J Proteome Res. 2024 Feb 2;23(2):749-759. doi: 10.1021/acs.jproteome.3c00629. Epub 2024 Jan 24. J Proteome Res. 2024. PMID: 38266179 Free PMC article.
-
The immunomodulating roles of glycoproteins in epithelial ovarian cancer.Front Biosci (Elite Ed). 2012 Jan 1;4(2):631-50. doi: 10.2741/405. Front Biosci (Elite Ed). 2012. PMID: 22201900 Free PMC article. Review.
-
Single-Cell Sequencing of Malignant Ascites Reveals Transcriptomic Remodeling of the Tumor Microenvironment during the Progression of Epithelial Ovarian Cancer.Genes (Basel). 2022 Dec 2;13(12):2276. doi: 10.3390/genes13122276. Genes (Basel). 2022. PMID: 36553542 Free PMC article.
-
Tumor Microenvironment-Associated Extracellular Matrix Components Regulate NK Cell Function.Front Immunol. 2020 Jan 29;11:73. doi: 10.3389/fimmu.2020.00073. eCollection 2020. Front Immunol. 2020. PMID: 32063906 Free PMC article. Review.
-
Tumor antigen CA125 suppresses antibody-dependent cellular cytotoxicity (ADCC) via direct antibody binding and suppressed Fc-γ receptor engagement.Oncotarget. 2017 Jul 7;8(32):52045-52060. doi: 10.18632/oncotarget.19090. eCollection 2017 Aug 8. Oncotarget. 2017. PMID: 28881712 Free PMC article.
References
-
- Carlsten M, Bjorkstrom NK, Norell H, Bryceson Y, van Hall T, Baumann BC, Hanson M, Schedvins K, Kiessling R, Ljunggren HG, Malmberg KJ. DNAX accessory molecule-1 mediated recognition of freshly isolated ovarian carcinoma by resting natural killer cells. Cancer Res. 2007;67:1317–1325. doi: 10.1158/0008-5472.CAN-06-2264. - DOI - PubMed
-
- Taylor DD, Gercel-Taylor C, Lyons KS, Stanson J, Whiteside TL. T-cell apoptosis and suppression of T-cell receptor/CD3-zeta by Fas ligand-containing membrane vesicles shed from ovarian tumors. Clin Cancer Res. 2003;9:5113–5119. - PubMed
-
- Lai P, Rabinowich H, Crowley-Nowick PA, Bell MC, Mantovani G, Whiteside TL. Alterations in expression and function of signal-transducing proteins in tumor-associated T and natural killer cells in patients with ovarian carcinoma. Clin Cancer Res. 1996;2:161–173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

