Analysis of retinal cell development in chick embryo by immunohistochemistry and in ovo electroporation techniques
- PMID: 20089190
- PMCID: PMC2822752
- DOI: 10.1186/1471-213X-10-8
Analysis of retinal cell development in chick embryo by immunohistochemistry and in ovo electroporation techniques
Abstract
Background: Retinal cell development has been extensively investigated; however, the current knowledge of dynamic morphological and molecular changes is not yet complete.
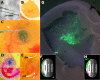
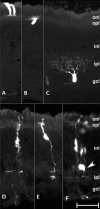
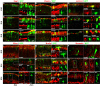
Results: This study was aimed at revealing the dynamic morphological and molecular changes in retinal cell development during the embryonic stages using a new method of targeted retinal injection, in ovo electroporation, and immunohistochemistry techniques. A plasmid DNA that expresses the green fluorescent protein (GFP) as a marker was delivered into the sub-retinal space to transfect the chick retinal stem/progenitor cells at embryonic day 3 (E3) or E4 with the aid of pulses of electric current. The transfected retinal tissues were analyzed at various stages during chick development from near the start of neurogenesis at E4 to near the end of neurogenesis at E18. The expression of GFP allowed for clear visualization of cell morphologies and retinal laminar locations for the indication of retinal cell identity. Immunohistochemistry using cell type-specific markers (e.g., Visinin, Xap-1, Lim1+2, Pkcalpha, NeuN, Pax6, Brn3a, Vimentin, etc.) allowed further confirmation of retinal cell types. The composition of retinal cell types was then determined over time by counting the number of GFP-expressing cells observed with morphological characteristics specific to the various retinal cell types.
Conclusion: The new method of retinal injection and electroporation at E3 - E4 allows the visualization of all retinal cell types, including the late-born neurons, e.g., bipolar cells at a level of single cells, which has been difficult with a conventional method with injection and electroporation at E1.5. Based on data collected from analyses of cell morphology, laminar locations in the retina, immunohistochemistry, and cell counts of GFP-expressing cells, the time-line and dynamic morphological and molecular changes of retinal cell development were determined. These data provide more complete information on retinal cell development, and they can serve as a reference for the investigations in normal retinal development and diseases.
Figures




Similar articles
-
In ovo electroporation in embryonic chick retina.J Vis Exp. 2012 Feb 5;(60):3792. doi: 10.3791/3792. J Vis Exp. 2012. PMID: 22330044 Free PMC article.
-
Ex vivo electroporation of retinal cells: a novel, high efficiency method for functional studies in primary retinal cultures.Exp Eye Res. 2013 Apr;109:40-50. doi: 10.1016/j.exer.2013.01.010. Epub 2013 Jan 28. Exp Eye Res. 2013. PMID: 23370269 Free PMC article.
-
Targeted gene expression in the chicken eye by in ovo electroporation.Mol Vis. 2004 Nov 17;10:874-83. Mol Vis. 2004. PMID: 15570216
-
Gain- and loss-of-function approaches in the chick embryo.Methods Cell Biol. 2008;87:237-56. doi: 10.1016/S0091-679X(08)00212-4. Methods Cell Biol. 2008. PMID: 18485300 Review.
-
A primer on using in ovo electroporation to analyze gene function.Dev Dyn. 2004 Mar;229(3):433-9. doi: 10.1002/dvdy.10473. Dev Dyn. 2004. PMID: 14991698 Review.
Cited by
-
Postnatal development of rat retina: a continuous observation and comparison between the organotypic retinal explant model and in vivo development.Neural Regen Res. 2025 Mar 1;20(3):900-912. doi: 10.4103/NRR.NRR-D-23-01557. Epub 2024 Apr 3. Neural Regen Res. 2025. PMID: 38886961 Free PMC article.
-
Influence of Different Light Spectra on Melatonin Synthesis by the Pineal Gland and Influence on the Immune System in Chickens.Animals (Basel). 2023 Jun 24;13(13):2095. doi: 10.3390/ani13132095. Animals (Basel). 2023. PMID: 37443893 Free PMC article. Review.
-
Horizontal cells expressing melanopsin x are novel photoreceptors in the avian inner retina.Proc Natl Acad Sci U S A. 2016 Nov 15;113(46):13215-13220. doi: 10.1073/pnas.1608901113. Epub 2016 Oct 27. Proc Natl Acad Sci U S A. 2016. PMID: 27789727 Free PMC article.
-
Neuroprotection by α2-Adrenergic Receptor Stimulation after Excitotoxic Retinal Injury: A Study of the Total Population of Retinal Ganglion Cells and Their Distribution in the Chicken Retina.PLoS One. 2016 Sep 9;11(9):e0161862. doi: 10.1371/journal.pone.0161862. eCollection 2016. PLoS One. 2016. PMID: 27611432 Free PMC article.
-
Identification of a transient Sox5 expressing progenitor population in the neonatal ventral forebrain by a novel cis-regulatory element.Dev Biol. 2014 Sep 1;393(1):183-93. doi: 10.1016/j.ydbio.2014.06.010. Epub 2014 Jun 19. Dev Biol. 2014. PMID: 24954155 Free PMC article.
References
-
- Kaneko A. Physiology of the retina. Annu Rev Neurosci. 1979;2:169–91. - PubMed
-
- Young RW. Cell differentiation in the retina of the mouse. Anat Rec. 1985;212:199–205. - PubMed
-
- Young RW. Cell proliferation during postnatal development of the retina in the mouse. Brain Res. 1985;353:229–39. - PubMed
-
- Alexiades MR, Cepko C. Quantitative analysis of proliferation and cell cycle length during development of the rat retina. Dev Dyn. 1996;205:293–307. - PubMed
-
- Gloor BP, Rokos L, Kaldarar-Pedotti S. Cell cycle time and life-span of cells in the mouse eye. Measurements during the postfetal period using repeated 3H-thymidine injections. Dev Ophthalmol. 1985;12:70–129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

