Targeted disruption of outer limiting membrane junctional proteins (Crb1 and ZO-1) increases integration of transplanted photoreceptor precursors into the adult wild-type and degenerating retina
- PMID: 20089206
- PMCID: PMC2938729
- DOI: 10.3727/096368909X486057
Targeted disruption of outer limiting membrane junctional proteins (Crb1 and ZO-1) increases integration of transplanted photoreceptor precursors into the adult wild-type and degenerating retina
Abstract
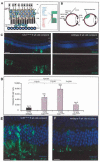
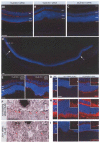
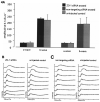
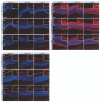
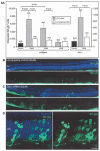
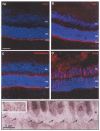
Diseases culminating in photoreceptor loss are a major cause of untreatable blindness. Transplantation of rod photoreceptors is feasible, provided donor cells are at an appropriate stage of development when transplanted. Nevertheless, the proportion of cells that integrate into the recipient outer nuclear layer (ONL) is low. The outer limiting membrane (OLM), formed by adherens junctions between Müller glia and photoreceptors, may impede transplanted cells from migrating into the recipient ONL. Adaptor proteins such as Crumbs homologue 1 (Crb1) and zona occludins (ZO-1) are essential for localization of the OLM adherens junctions. We investigated whether targeted disruption of these proteins enhances donor cell integration. Transplantation of rod precursors in wild-type mice achieved 949 +/- 141 integrated cells. By contrast, integration is significantly higher when rod precursors are transplanted into Crb1(rd8/rd8) mice, a model of retinitis pigmentosa and Lebers congenital amaurosis that lacks functional CRB1 protein and displays disruption of the OLM (7,819 +/- 1,297; maximum 15,721 cells). We next used small interfering (si)RNA to transiently reduce the expression of ZO-1 and generate a reversible disruption of the OLM. ZO-1 knockdown resulted in similar, significantly improved, integration of transplanted cells in wild-type mice (7,037 +/- 1,293; maximum 11,965 cells). Finally, as the OLM remains largely intact in many retinal disorders, we tested whether transient ZO-1 knockdown increased integration in a model of retinitis pigmentosa, the rho(-/-) mouse; donor cell integration was significantly increased from 313 +/- 58 cells without treatment to 919 +/- 198 cells after ZO-1 knockdown. This study shows that targeted disruption of OLM junctional proteins enhances integration in the wild-type and degenerating retina and may be a useful approach for developing photoreceptor transplantation strategies.
Figures

References
-
- Aijaz S, Balda MS, Matter K. Tight junctions: Molecular architecture and function. Int. Rev. Cytol. 2006;248:261–298. - PubMed
-
- Akimoto M, Cheng H, Zhu D, Brzezinski JA, Filippova E, Oh ECT, Jing Y, Linares J-L, Brooks M, Zareparsi S, Mears AJ, Hero A, Glaser T, Swaroop A. Targeting of green fluorescent protein to new-born rods by Nrl promoter and temporal expression profiling of flow-soreted photoreceptors. Proc. Natl. Acad. Sci. USA. 2006;103(10):3890–3895. - PMC - PubMed
-
- Bartsch U, Oriyakhel W, Kenna PF, Linke S, Richard G, Petrowitz B, Humphries P, Farrar GJ, Ader M. Retinal cells integrate into the outer nuclear layer and differentiate into mature photoreceptors after subretinal transplantation into adult mice. Exp. Eye Res. 2008;86:691–700. - PubMed
-
- Campbell M, Humphries M, Kenna P, Humphries P, Brankin B. Altered expression and interaction of adherens junction proteins in the developing OLM of the Rho(−/−) mouse. Exp. Eye Res. 2007;85:714–720. - PubMed
-
- Campbell M, Humphries M, Kennan A, Kenna P, Humphries P, Brankin B. Aberrant retinal tight junction and adherens junction protein expression in an animal model of autosomal dominant Retinitis pigmentosa: The Rho(−/−) mouse. Exp. Eye Res. 2006;83:484–492. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

