Glycoprotein D actively induces rapid internalization of two nectin-1 isoforms during herpes simplex virus entry
- PMID: 20089288
- PMCID: PMC2830393
- DOI: 10.1016/j.virol.2009.12.034
Glycoprotein D actively induces rapid internalization of two nectin-1 isoforms during herpes simplex virus entry
Abstract
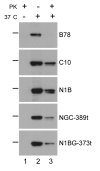
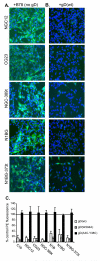
Entry of herpes simplex virus (HSV) occurs either by fusion at the plasma membrane or by endocytosis and fusion with an endosome. Binding of glycoprotein D (gD) to a receptor such as nectin-1 is essential in both cases. We show that virion gD triggered the rapid down-regulation of nectin-1 with kinetics similar to those of virus entry. In contrast, nectin-1 was not constitutively recycled from the surface of uninfected cells. Both the nectin-1alpha and beta isoforms were internalized in response to gD despite having different cytoplasmic tails. However, deletion of the nectin-1 cytoplasmic tail slowed down-regulation of nectin-1 and internalization of virions. These data suggest that nectin-1 interaction with a cytoplasmic protein is not required for its down-regulation. Overall, this study shows that gD binding actively induces the rapid internalization of various forms of nectin-1. We suggest that HSV activates a nectin-1 internalization pathway to use for endocytic entry.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
The herpes simplex virus receptor nectin-1 is down-regulated after trans-interaction with glycoprotein D.Virology. 2008 Mar 30;373(1):98-111. doi: 10.1016/j.virol.2007.11.012. Epub 2008 Feb 20. Virology. 2008. PMID: 18076965 Free PMC article.
-
Cbl E3 Ligase Mediates the Removal of Nectin-1 from the Surface of Herpes Simplex Virus 1-Infected Cells.J Virol. 2017 May 26;91(12):e00393-17. doi: 10.1128/JVI.00393-17. Print 2017 Jun 15. J Virol. 2017. PMID: 28381567 Free PMC article.
-
Mutations in the N termini of herpes simplex virus type 1 and 2 gDs alter functional interactions with the entry/fusion receptors HVEM, nectin-2, and 3-O-sulfated heparan sulfate but not with nectin-1.J Virol. 2003 Sep;77(17):9221-31. doi: 10.1128/jvi.77.17.9221-9231.2003. J Virol. 2003. PMID: 12915538 Free PMC article.
-
Two Sides to Every Story: Herpes Simplex Type-1 Viral Glycoproteins gB, gD, gH/gL, gK, and Cellular Receptors Function as Key Players in Membrane Fusion.Viruses. 2021 Sep 16;13(9):1849. doi: 10.3390/v13091849. Viruses. 2021. PMID: 34578430 Free PMC article. Review.
-
Receptors and ligands for herpes simplex viruses: Novel insights for drug targeting.Drug Discov Today. 2022 Jan;27(1):185-195. doi: 10.1016/j.drudis.2021.10.004. Epub 2021 Oct 19. Drug Discov Today. 2022. PMID: 34678489 Review.
Cited by
-
Herpes simplex virus glycoprotein D relocates nectin-1 from intercellular contacts.Virology. 2016 Dec;499:267-277. doi: 10.1016/j.virol.2016.09.019. Epub 2016 Oct 7. Virology. 2016. PMID: 27723487 Free PMC article.
-
Cascade of events governing cell-cell fusion induced by herpes simplex virus glycoproteins gD, gH/gL, and gB.J Virol. 2010 Dec;84(23):12292-9. doi: 10.1128/JVI.01700-10. Epub 2010 Sep 22. J Virol. 2010. PMID: 20861251 Free PMC article.
-
Downregulation of endogenous nectin1 in human keratinocytes by herpes simplex virus 1 glycoprotein D excludes superinfection but does not affect NK cell function.J Gen Virol. 2024 Mar;105(3):001969. doi: 10.1099/jgv.0.001969. J Gen Virol. 2024. PMID: 38471041 Free PMC article.
-
Chlamydial Pre-Infection Protects from Subsequent Herpes Simplex Virus-2 Challenge in a Murine Vaginal Super-Infection Model.PLoS One. 2016 Jan 4;11(1):e0146186. doi: 10.1371/journal.pone.0146186. eCollection 2016. PLoS One. 2016. PMID: 26726882 Free PMC article.
-
DNAM-1 Activating Receptor and Its Ligands: How Do Viruses Affect the NK Cell-Mediated Immune Surveillance during the Various Phases of Infection?Int J Mol Sci. 2019 Jul 30;20(15):3715. doi: 10.3390/ijms20153715. Int J Mol Sci. 2019. PMID: 31366013 Free PMC article. Review.
References
-
- Cocchi F, Menotti L, Mirandola P, Lopez M, Campadelli-Fiume G. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attribute of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J. Virol. 1998;72:9992–10002. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56 AI076231/AI/NIAID NIH HHS/United States
- R01 AI076231/AI/NIAID NIH HHS/United States
- AI-076231/AI/NIAID NIH HHS/United States
- AI-073384/AI/NIAID NIH HHS/United States
- AI-056045/AI/NIAID NIH HHS/United States
- R21 AI073384/AI/NIAID NIH HHS/United States
- R01 AI018289/AI/NIAID NIH HHS/United States
- R21 AI056045/AI/NIAID NIH HHS/United States
- T32-AI07324/AI/NIAID NIH HHS/United States
- T32 AI007324/AI/NIAID NIH HHS/United States
- AI-18289/AI/NIAID NIH HHS/United States
- R01 AI056045/AI/NIAID NIH HHS/United States
- R37 AI018289/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

