Randomized, double-blind, controlled study of losartan in children with proteinuria
- PMID: 20089489
- PMCID: PMC2827569
- DOI: 10.2215/CJN.06620909
Randomized, double-blind, controlled study of losartan in children with proteinuria
Abstract
Background and objectives: No large, randomized, double-blind trials in children with proteinuria treated with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers have previously been reported.
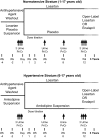
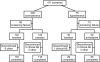
Design, setting, participants, & measurements: This 12-week, double-blind, multinational study investigated the effects of losartan 0.7 to 1.4 mg/kg per day compared with placebo (normotensive stratum) or amlodipine 0.1 to 0.2 mg/kg per day up to 5 mg/d (hypertensive stratum) on proteinuria (morning-void urinary protein-creatinine ratio, baseline > or =0.3 g/g) in 306 children up to 17 years of age.
Results: Twelve weeks of treatment with losartan significantly reduced proteinuria compared with amlodipine/placebo: losartan -35.8% (95% confidence interval: -27.6% to -43.1%) versus amlodipine/placebo 1.4% (95% confidence interval: -10.3% to 14.5%), P < or = 0.001. Significance remained after adjustment for differences across treatment groups in change in BP (losartan produced incremental systolic and diastolic BP reductions versus amlodipine of 5.4 and 4.6 mmHg, respectively; and versus placebo of 3.8 and 4.0 mmHg, respectively). Proteinuria reduction was consistently observed in the normotensive (-34.4% losartan; 2.6% placebo) and hypertensive (-41.5% losartan; 2.4% amlodipine) strata, and in all prespecified subgroups, including age, gender, race, Tanner stage, weight, prior therapy with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, as well as among the most common etiologies of proteinuria. Adverse event incidence was low and comparable in all groups.
Conclusions: Losartan significantly lowered proteinuria and was well tolerated after 12 weeks in children aged 1 to 17 years with proteinuria with or without hypertension, a population that has not previously been rigorously studied.
Figures
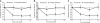


References
-
- Wong H, Mylrea K, Feber J, Drukker A, Filler G: Prevalence of complications in children with chronic kidney disease according to KDOQI. Kidney Int 70: 585–590, 2006 - PubMed
-
- Hogg RJ, Portman RJ, Milliner D, Lemley KV, Eddy A, Ingelfinger J: Evaluation and management of proteinuria and nephrotic syndrome in children: Recommendations from a pediatric nephrology panel established at the National Kidney Foundation conference on proteinuria, albuminuria, risk, assessment, detection, and elimination (PARADE). Pediatrics 105: 1242–1249, 2000 - PubMed
-
- Remuzzi G, Ruggenenti P, Benigni A: Understanding the nature of renal disease progression. Kidney Int 51: 2–15, 1997 - PubMed
-
- Grimm RH, Jr., Svendsen KH, Kasiske B, Keane WF, Wahi MM: Proteinuria is a risk factor for mortality over 10 years of follow-up. MRFIT Research Group. Multiple Risk Factor Intervention Trial. Kidney Int ( Suppl) 63: S10–S14, 1997 - PubMed
-
- Portman R, Hawkins E, Verani R: Premature atherosclerosis (PA) in pediatric renal patients (PRP): Report of the southwest pediatric nephrology study group [Abstract]. Pediatr Res 29: 349A, 1991
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

