Structural and mechanistic insights into Helicobacter pylori NikR activation
- PMID: 20089510
- PMCID: PMC2875016
- DOI: 10.1093/nar/gkp1216
Structural and mechanistic insights into Helicobacter pylori NikR activation
Abstract
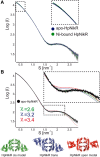
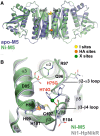
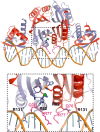
NikR is a transcriptional metalloregulator central in the mandatory response to acidity of Helicobacter pylori that controls the expression of numerous genes by binding to specific promoter regions. NikR/DNA interactions were proposed to rely on protein activation by Ni(II) binding to high-affinity (HA) and possibly secondary external (X) sites. We describe a biochemical characterization of HpNikR mutants that shows that the HA sites are essential but not sufficient for DNA binding, while the secondary external (X) sites and residues from the HpNikR dimer-dimer interface are important for DNA binding. We show that a second metal is necessary for HpNikR/DNA binding, but only to some promoters. Small-angle X-ray scattering shows that HpNikR adopts a defined conformation in solution, resembling the cis-conformation and suggests that nickel does not trigger large conformational changes in HpNikR. The crystal structures of selected mutants identify the effects of each mutation on HpNikR structure. This study unravels key structural features from which we derive a model for HpNikR activation where: (i) HA sites and an hydrogen bond network are required for DNA binding and (ii) metallation of a unique secondary external site (X) modulates HpNikR DNA binding to low-affinity promoters by disruption of a salt bridge.
Figures







Similar articles
-
Ni(II) coordination to mixed sites modulates DNA binding of HpNikR via a long-range effect.Proc Natl Acad Sci U S A. 2012 Apr 10;109(15):5633-8. doi: 10.1073/pnas.1120283109. Epub 2012 Mar 26. Proc Natl Acad Sci U S A. 2012. PMID: 22451934 Free PMC article.
-
pH-responsive DNA-binding activity of Helicobacter pylori NikR.Biochemistry. 2009 Mar 24;48(11):2486-96. doi: 10.1021/bi801742r. Biochemistry. 2009. PMID: 19170600
-
High-affinity Ni2+ binding selectively promotes binding of Helicobacter pylori NikR to its target urease promoter.J Mol Biol. 2008 Nov 28;383(5):1129-43. doi: 10.1016/j.jmb.2008.08.066. Epub 2008 Sep 4. J Mol Biol. 2008. PMID: 18790698
-
Helicobacter pylori NikR protein exhibits distinct conformations when bound to different promoters.J Biol Chem. 2011 May 6;286(18):15728-37. doi: 10.1074/jbc.M110.196055. Epub 2011 Mar 10. J Biol Chem. 2011. PMID: 21393642 Free PMC article.
-
Insights into the Allosteric Response to Acidity by the Helicobacter pylori NikR Transcription Factor.Biochemistry. 2023 Nov 21;62(22):3265-3275. doi: 10.1021/acs.biochem.3c00356. Epub 2023 Nov 2. Biochemistry. 2023. PMID: 37917856
Cited by
-
Bacterial Transcriptional Regulators: A Road Map for Functional, Structural, and Biophysical Characterization.Int J Mol Sci. 2022 Feb 16;23(4):2179. doi: 10.3390/ijms23042179. Int J Mol Sci. 2022. PMID: 35216300 Free PMC article. Review.
-
Nickel Metalloregulators and Chaperones.Inorganics (Basel). 2019 Aug;7(8):10.3390/inorganics7080104. doi: 10.3390/inorganics7080104. Epub 2019 Aug 19. Inorganics (Basel). 2019. PMID: 32133383 Free PMC article.
-
Insights into the Orchestration of Gene Transcription Regulators in Helicobacter pylori.Int J Mol Sci. 2022 Nov 8;23(22):13688. doi: 10.3390/ijms232213688. Int J Mol Sci. 2022. PMID: 36430169 Free PMC article. Review.
-
Evolution of Macromolecular Docking Techniques: The Case Study of Nickel and Iron Metabolism in Pathogenic Bacteria.Molecules. 2015 Aug 5;20(8):14265-92. doi: 10.3390/molecules200814265. Molecules. 2015. PMID: 26251891 Free PMC article. Review.
-
In vivo recognition of the fecA3 target promoter by Helicobacter pylori NikR.J Bacteriol. 2011 Mar;193(5):1131-41. doi: 10.1128/JB.01153-10. Epub 2011 Jan 7. J Bacteriol. 2011. PMID: 21216998 Free PMC article.
References
-
- Waldron KJ, Robinson NJ. How do bacterial cells ensure that metalloproteins get the correct metal? Nat. Rev. Microbiol. 2009;7:25–35. - PubMed
-
- Dosanjh NS, Michel SL. Microbial nickel metalloregulation: NikRs for nickel ions. Curr. Opin. Chem. Biol. 2006;10:123–130. - PubMed
-
- Clyne M, Dolan B, Reeves EP. Bacterial factors that mediate colonization of the stomach and virulence of Helicobacter pylori. FEMS Microbiol. Lett. 2007;268:135–143. - PubMed
-
- de Reuse H, Bereswill S. Ten years after the first Helicobacter pylori genome: comparative and functional genomics provide new insights in the variability and adaptability of a persistent pathogen. FEMS Immunol. Med. Microbiol. 2007;50:165–176. - PubMed

