12-Lipoxygenase Products Reduce Insulin Secretion and {beta}-Cell Viability in Human Islets
- PMID: 20089617
- PMCID: PMC2840856
- DOI: 10.1210/jc.2009-1102
12-Lipoxygenase Products Reduce Insulin Secretion and {beta}-Cell Viability in Human Islets
Abstract
Context: Inflammation is increasingly recognized as an important contributing factor in diabetes mellitus. Lipoxygenases (LOs) produce active lipids that promote inflammatory damage by catalyzing the oxidation of linoleic and arachidonic acid, and LO is expressed in rodent and human islets. Little is known about the differential effect of the various hydroxyeicosatetraenoic acids (HETEs) that result from LO activity in human islets.
Objective: We compared the effects of 12-LO products on human islet viability and function.
Design: Human islets were treated with stable compounds derived from LOs: 12(S)-HETE, 15HETE, 12HPETE, and 12RHETE and then examined for insulin secretion and islet viability. The p38-MAPK (p38) and JNK stress-activated pathways were investigated as mechanisms of 12-LO-mediated islet inhibition in rodent and human islets.
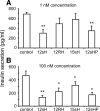
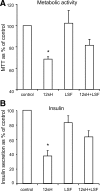
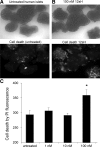
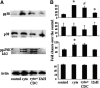
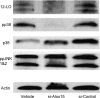
Results: Insulin secretion was consistently reduced by 12(S)-HETE and 12HPETE. 12(S)-HETE at 1 nm reduced viability activity by 32% measured by MTT assay and increased cell death by 50% at 100 nm in human islets. These effects were partially reversed with lisofylline, a small-molecule antiinflammatory compound that protects mitochondrial function. 12(S)-HETE increased phosphorylated p38-MAPK (pp38) protein activity in human islets. Injecting 12-LO siRNA into C57BL/6 mice reduced 12-LO and pp38-MAPK protein levels in mouse islets. The addition of proinflammatory cytokines increased pp38 levels in normal mouse islets but not in siRNA-treated islets.
Conclusions: These data suggest that 12(S)-HETE reduces insulin secretion and increases cell death in human islets. The 12-LO pathway is present in human islets, and expression is up-regulated by inflammatory cytokines. Reduction of 12-LO activity could thus provide a new therapeutic approach to protect human beta-cells from inflammatory injury.
Figures
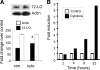
Similar articles
-
Selective inhibition of 12-lipoxygenase protects islets and beta cells from inflammatory cytokine-mediated beta cell dysfunction.Diabetologia. 2015 Mar;58(3):549-57. doi: 10.1007/s00125-014-3452-0. Epub 2014 Nov 23. Diabetologia. 2015. PMID: 25417214
-
Activation of 12-lipoxygenase in proinflammatory cytokine-mediated beta cell toxicity.Diabetologia. 2005 Mar;48(3):486-95. doi: 10.1007/s00125-005-1673-y. Epub 2005 Feb 24. Diabetologia. 2005. PMID: 15729574
-
Evidence that increased 12-lipoxygenase expression impairs pancreatic beta cell function and viability.Biochem Biophys Res Commun. 2003 Aug 29;308(3):427-32. doi: 10.1016/s0006-291x(03)01418-9. Biochem Biophys Res Commun. 2003. PMID: 12914766
-
Lipids and immunoinflammatory pathways of beta cell destruction.Diabetologia. 2016 Apr;59(4):673-8. doi: 10.1007/s00125-016-3890-y. Epub 2016 Feb 11. Diabetologia. 2016. PMID: 26868492 Free PMC article. Review.
-
The role of 12-lipoxygenase in pancreatic -cells (Review).Int J Mol Med. 1998 Jan;1(1):265-72. Int J Mol Med. 1998. PMID: 9852229 Review.
Cited by
-
Structural and functional biology of arachidonic acid 15-lipoxygenase-1 (ALOX15).Gene. 2015 Nov 15;573(1):1-32. doi: 10.1016/j.gene.2015.07.073. Epub 2015 Jul 26. Gene. 2015. PMID: 26216303 Free PMC article. Review.
-
Concordance of changes in metabolic pathways based on plasma metabolomics and skeletal muscle transcriptomics in type 1 diabetes.Diabetes. 2012 May;61(5):1004-16. doi: 10.2337/db11-0874. Epub 2012 Mar 13. Diabetes. 2012. PMID: 22415876 Free PMC article.
-
The emerging role of oxylipins in thrombosis and diabetes.Front Pharmacol. 2014 Jan 7;4:176. doi: 10.3389/fphar.2013.00176. eCollection 2014 Jan 7. Front Pharmacol. 2014. PMID: 24432004 Free PMC article. Review.
-
STAT4 deficiency reduces obesity-induced insulin resistance and adipose tissue inflammation.Diabetes. 2013 Dec;62(12):4109-21. doi: 10.2337/db12-1275. Epub 2013 Aug 12. Diabetes. 2013. PMID: 23939393 Free PMC article.
-
12-Lipoxygenase Regulates Cold Adaptation and Glucose Metabolism by Producing the Omega-3 Lipid 12-HEPE from Brown Fat.Cell Metab. 2019 Oct 1;30(4):768-783.e7. doi: 10.1016/j.cmet.2019.07.001. Epub 2019 Jul 25. Cell Metab. 2019. PMID: 31353262 Free PMC article.
References
-
- Yamamoto S 1992 Mammalian lipoxygenase: molecular structures and functions. Biochim Biophys Acta 1128:117–131 - PubMed
-
- Funk CD 1996 The molecular biology of mammalian lipoxygenase and the quest for eicosanoid functions using lipoxygenase-deficient mice. Biochim Biophys Acta 1304:65–84 - PubMed
-
- Bleich D, Chen S, Gu JL, Nadler JL 1998 The role of 12-lipoxygenase in pancreatic-cells (review). Int J Mol Med 1:265–272 - PubMed
-
- McDuffie M, Maybee NA, Keller SR, Stevens BK, Garmey JC, Morris MA, Kropf E, Rival C, Ma K, Carter JD, Tersey SA, Nunemaker CS, Nadler JL 2008 Nonobese diabetic (NOD) mice congenic for a targeted deletion of 12/15-lipoxygenase are protected from autoimmune diabetes. Diabetes 57:199–208 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

