A V3 loop-dependent gp120 element disrupted by CD4 binding stabilizes the human immunodeficiency virus envelope glycoprotein trimer
- PMID: 20089638
- PMCID: PMC2838131
- DOI: 10.1128/JVI.02587-09
A V3 loop-dependent gp120 element disrupted by CD4 binding stabilizes the human immunodeficiency virus envelope glycoprotein trimer
Abstract
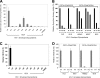
Human immunodeficiency virus (HIV-1) entry into cells is mediated by a trimeric complex consisting of noncovalently associated gp120 (exterior) and gp41 (transmembrane) envelope glycoproteins. The binding of gp120 to receptors on the target cell alters the gp120-gp41 relationship and activates the membrane-fusing capacity of gp41. Interaction of gp120 with the primary receptor, CD4, results in the exposure of the gp120 third variable (V3) loop, which contributes to binding the CCR5 or CXCR4 chemokine receptors. We show here that insertions in the V3 stem or polar substitutions in a conserved hydrophobic patch near the V3 tip result in decreased gp120-gp41 association (in the unliganded state) and decreased chemokine receptor binding (in the CD4-bound state). Subunit association and syncytium-forming ability of the envelope glycoproteins from primary HIV-1 isolates were disrupted more by V3 changes than those of laboratory-adapted HIV-1 envelope glycoproteins. Changes in the gp120 beta2, beta19, beta20, and beta21 strands, which evidence suggests are proximal to the V3 loop in unliganded gp120, also resulted in decreased gp120-gp41 association. Thus, a gp120 element composed of the V3 loop and adjacent beta strands contributes to quaternary interactions that stabilize the unliganded trimer. CD4 binding dismantles this element, altering the gp120-gp41 relationship and rendering the hydrophobic patch in the V3 tip available for chemokine receptor binding.
Figures





Similar articles
-
Activation and Inactivation of Primary Human Immunodeficiency Virus Envelope Glycoprotein Trimers by CD4-Mimetic Compounds.J Virol. 2017 Jan 18;91(3):e01880-16. doi: 10.1128/JVI.01880-16. Print 2017 Feb 1. J Virol. 2017. PMID: 27881646 Free PMC article.
-
Structural basis of coreceptor recognition by HIV-1 envelope spike.Nature. 2019 Jan;565(7739):318-323. doi: 10.1038/s41586-018-0804-9. Epub 2018 Dec 12. Nature. 2019. PMID: 30542158 Free PMC article.
-
Residues in the gp41 Ectodomain Regulate HIV-1 Envelope Glycoprotein Conformational Transitions Induced by gp120-Directed Inhibitors.J Virol. 2017 Feb 14;91(5):e02219-16. doi: 10.1128/JVI.02219-16. Print 2017 Mar 1. J Virol. 2017. PMID: 28003492 Free PMC article.
-
CD4 activation of HIV fusion.Int J Cell Cloning. 1992 Nov;10(6):323-32. doi: 10.1002/stem.5530100603. Int J Cell Cloning. 1992. PMID: 1281202 Review.
-
Conformational properties of HIV-1 gp120/V3 immunogenic domains.Curr Med Chem. 2005;12(13):1551-68. doi: 10.2174/0929867054038982. Curr Med Chem. 2005. PMID: 15974987 Review.
Cited by
-
Alterations in gp120 glycans or the gp41 fusion peptide-proximal region modulate the stability of the human immunodeficiency virus (HIV-1) envelope glycoprotein pretriggered conformation.J Virol. 2023 Sep 28;97(9):e0059223. doi: 10.1128/jvi.00592-23. Epub 2023 Sep 11. J Virol. 2023. PMID: 37696048 Free PMC article.
-
Visualization of retroviral envelope spikes in complex with the V3 loop antibody 447-52D on intact viruses by cryo-electron tomography.J Virol. 2014 Nov;88(21):12265-75. doi: 10.1128/JVI.01596-14. Epub 2014 Aug 13. J Virol. 2014. PMID: 25122783 Free PMC article.
-
Novel small synthetic HIV-1 V3 crown variants: CCR5 targeting ligands.J Biochem. 2022 Sep 5;172(3):149-164. doi: 10.1093/jb/mvac052. J Biochem. 2022. PMID: 35708645 Free PMC article.
-
Assessing bnAb potency in the context of HIV-1 envelope conformational plasticity.PLoS Pathog. 2025 Jan 21;21(1):e1012825. doi: 10.1371/journal.ppat.1012825. eCollection 2025 Jan. PLoS Pathog. 2025. PMID: 39836706 Free PMC article.
-
Dynamics of envelope evolution in clade C SHIV-infected pig-tailed macaques during disease progression analyzed by ultra-deep pyrosequencing.PLoS One. 2012;7(3):e32827. doi: 10.1371/journal.pone.0032827. Epub 2012 Mar 12. PLoS One. 2012. PMID: 22427893 Free PMC article.
References
-
- Alkhatib, G., C. Combadiere, C. C. Broder, Y. Feng, P. E. Kennedy, P. M. Murphy, and E. A. Berger. 1996. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955-1958. - PubMed
-
- Babcock, G., T. Mirzabekov, W. Wojtowicz, and J. Sodroski. 2001. Ligand binding characteristics of CXCR4 incorporated into paramagnetic proteoliposomes. J. Biol. Chem. 276:38433-38440. - PubMed
-
- Baik, S. S., R. W. Doms, and B. J. Doranz. 1999. HIV and SIV gp120 binding does not predict coreceptor function. Virology 259:267-273. - PubMed
-
- Chen, B., E. M. Vogan, H. Gong, J. J. Skehel, D. C. Wiley, and S. C. Harrison. 2005. Structure of an unliganded simian immunodeficiency virus gp120 core. Nature 433:834-841. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

