Extralymphoid CD8+ T cells resident in tissue from simian immunodeficiency virus SIVmac239{Delta}nef-vaccinated macaques suppress SIVmac239 replication ex vivo
- PMID: 20089651
- PMCID: PMC2838091
- DOI: 10.1128/JVI.02028-09
Extralymphoid CD8+ T cells resident in tissue from simian immunodeficiency virus SIVmac239{Delta}nef-vaccinated macaques suppress SIVmac239 replication ex vivo
Abstract
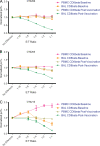
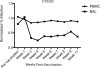
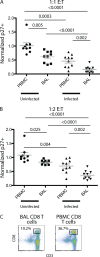
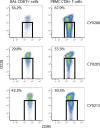
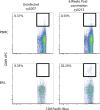
Live-attenuated vaccination with simian immunodeficiency virus (SIV) SIVmac239Deltanef is the most successful vaccine product tested to date in macaques. However, the mechanisms that explain the efficacy of this vaccine remain largely unknown. We utilized an ex vivo viral suppression assay to assess the quality of the immune response in SIVmac239Deltanef-immunized animals. Using major histocompatibility complex-matched Mauritian cynomolgus macaques, we did not detect SIV-specific functional immune responses in the blood by gamma interferon (IFN-gamma) enzyme-linked immunospot assay at select time points; however, we found that lung CD8(+) T cells, unlike blood CD8(+) T cells, effectively suppress virus replication by up to 80%. These results suggest that SIVmac239Deltanef may be an effective vaccine because it elicits functional immunity at mucosal sites. Moreover, these results underscore the limitations of relying on immunological measurements from peripheral blood lymphocytes in studies of protective immunity to HIV/SIV.
Figures



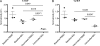
Similar articles
-
Inhibition of simian immunodeficiency virus (SIV) replication by CD8(+) T lymphocytes from macaques immunized with live attenuated SIV.J Virol. 1998 Aug;72(8):6315-24. doi: 10.1128/JVI.72.8.6315-6324.1998. J Virol. 1998. PMID: 9658070 Free PMC article.
-
The Frequency of Vaccine-Induced T-Cell Responses Does Not Predict the Rate of Acquisition after Repeated Intrarectal SIVmac239 Challenges in Mamu-B*08+ Rhesus Macaques.J Virol. 2019 Feb 19;93(5):e01626-18. doi: 10.1128/JVI.01626-18. Print 2019 Mar 1. J Virol. 2019. PMID: 30541854 Free PMC article.
-
Mamu-B*17+ Rhesus Macaques Vaccinated with env, vif, and nef Manifest Early Control of SIVmac239 Replication.J Virol. 2018 Jul 31;92(16):e00690-18. doi: 10.1128/JVI.00690-18. Print 2018 Aug 15. J Virol. 2018. PMID: 29875239 Free PMC article.
-
Both mucosal and systemic routes of immunization with the live, attenuated NYVAC/simian immunodeficiency virus SIV(gpe) recombinant vaccine result in gag-specific CD8(+) T-cell responses in mucosal tissues of macaques.J Virol. 2002 Nov;76(22):11659-76. doi: 10.1128/jvi.76.22.11659-11676.2002. J Virol. 2002. PMID: 12388726 Free PMC article.
-
Antiviral CD8+ T cells in the genital tract control viral replication and delay progression to AIDS after vaginal SIV challenge in rhesus macaques immunized with virulence attenuated SHIV 89.6.J Intern Med. 2009 Jan;265(1):67-77. doi: 10.1111/j.1365-2796.2008.02051.x. J Intern Med. 2009. PMID: 19093961 Free PMC article. Review.
Cited by
-
Expansion of Simian Immunodeficiency Virus (SIV)-Specific CD8 T Cell Lines from SIV-Naive Mauritian Cynomolgus Macaques for Adoptive Transfer.J Virol. 2015 Oct;89(19):9748-57. doi: 10.1128/JVI.00993-15. Epub 2015 Jul 15. J Virol. 2015. PMID: 26178985 Free PMC article.
-
Conditional CD8+ T cell escape during acute simian immunodeficiency virus infection.J Virol. 2012 Jan;86(1):605-9. doi: 10.1128/JVI.05511-11. Epub 2011 Oct 19. J Virol. 2012. PMID: 22013056 Free PMC article.
-
Gag-specific cellular immunity determines in vitro viral inhibition and in vivo virologic control following simian immunodeficiency virus challenges of vaccinated rhesus monkeys.J Virol. 2012 Sep;86(18):9583-9. doi: 10.1128/JVI.00996-12. Epub 2012 Jul 3. J Virol. 2012. PMID: 22761379 Free PMC article.
-
T-cell correlates of vaccine efficacy after a heterologous simian immunodeficiency virus challenge.J Virol. 2010 May;84(9):4352-65. doi: 10.1128/JVI.02365-09. Epub 2010 Feb 17. J Virol. 2010. PMID: 20164222 Free PMC article.
-
Magnetically enhanced nucleic acid delivery. Ten years of magnetofection-progress and prospects.Adv Drug Deliv Rev. 2011 Nov;63(14-15):1300-31. doi: 10.1016/j.addr.2011.08.002. Epub 2011 Aug 26. Adv Drug Deliv Rev. 2011. PMID: 21893135 Free PMC article. Review.
References
-
- Almond, N., K. Kent, M. Cranage, E. Rud, B. Clarke, and E. J. Stott. 1995. Protection by attenuated simian immunodeficiency virus in macaques against challenge with virus-infected cells. Lancet 345:1342-1344. - PubMed
-
- Almond, N., J. Rose, R. Sangster, P. Silvera, R. Stebbings, B. Walker, and E. J. Stott. 1997. Mechanisms of protection induced by attenuated simian immunodeficiency virus. I. Protection cannot be transferred with immune serum. J. Gen. Virol. 78:1919-1922. - PubMed
-
- Berry, N., R. Stebbings, D. Ferguson, C. Ham, J. Alden, S. Brown, A. Jenkins, J. Lines, L. Duffy, L. Davis, W. Elsley, M. Page, R. Hull, J. Stott, and N. Almond. 2008. Resistance to superinfection by a vigorously replicating, uncloned stock of simian immunodeficiency virus (SIVmac251) stimulates replication of a live attenuated virus vaccine (SIVmacC8). J. Gen. Virol. 89:2240-2251. - PubMed
-
- Brenchley, J. M., K. S. Knox, A. I. Asher, D. A. Price, L. M. Kohli, E. Gostick, B. J. Hill, C. A. Hage, Z. Brahmi, A. Khoruts, H. L. Twigg III, T. W. Schacker, and D. C. Douek. 2008. High frequencies of polyfunctional HIV-specific T cells are associated with preservation of mucosal CD4 T cells in bronchoalveolar lavage. Mucosal Immunol. 1:49-58. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

