Hepatitis B virus (HBV) surface antigen interacts with and promotes cyclophilin a secretion: possible link to pathogenesis of HBV infection
- PMID: 20089655
- PMCID: PMC2838095
- DOI: 10.1128/JVI.02555-09
Hepatitis B virus (HBV) surface antigen interacts with and promotes cyclophilin a secretion: possible link to pathogenesis of HBV infection
Abstract
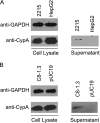
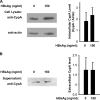
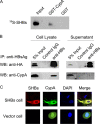
Cyclophilin A (CypA), predominantly located intracellularly, is a multifunctional protein. We previously reported decreased CypA levels in hepatocytes of transgenic mice expressing hepatitis B virus (HBV) surface antigen (HBsAg). In this study, we found that expression of HBV small surface protein (SHBs) in human hepatoma cell lines specifically triggered CypA secretion, whereas SHBs added extracellularly to culture medium did not. Moreover, CypA secretion was not promoted by the expression of a secretion deficient SHBs mutant, suggesting a close association between secretion of CypA and SHBs. Interaction between CypA and SHBs was observed by using coimmunoprecipitation and glutathione S-transferase pull-down assays. Hydrodynamic injection of the SHBs expression construct into C57BL/6J mice resulted in increased serum CypA levels and ALT/AST levels, as well as the infiltration of inflammatory cells surrounding SHBs-positive hepatocytes. The inflammatory response and serum ALT/AST level were reduced when the chemotactic effect of CypA was inhibited by cyclosporine and anti-CD147 antibody. Furthermore, higher serum CypA levels were detected in chronic hepatitis B patients than in healthy individuals. In HBV patients who had received liver transplantation, serum CypA levels declined dramatically after the loss of HBsAg as a consequence of liver transplantation. Taken together, these results indicate that expression and secretion of SHBs can promote CypA secretion, which may contribute to the pathogenesis of HBV infection.
Figures






Similar articles
-
Hepatitis B Virus Small Envelope Protein Promotes Hepatocellular Carcinoma Angiogenesis via Endoplasmic Reticulum Stress Signaling To Upregulate the Expression of Vascular Endothelial Growth Factor A.J Virol. 2022 Feb 23;96(4):e0197521. doi: 10.1128/JVI.01975-21. Epub 2021 Dec 15. J Virol. 2022. PMID: 34910612 Free PMC article.
-
Effects of amino acid substitutions in hepatitis B virus surface protein on virion secretion, antigenicity, HBsAg and viral DNA.J Hepatol. 2017 Feb;66(2):288-296. doi: 10.1016/j.jhep.2016.09.005. Epub 2016 Sep 17. J Hepatol. 2017. PMID: 27650283 Free PMC article.
-
Small hepatitis B virus surface antigen (SHBs) induces dyslipidemia by suppressing apolipoprotein-AII expression through ER stress-mediated modulation of HNF4α and C/EBPγ.J Virol. 2024 Nov 19;98(11):e0123924. doi: 10.1128/jvi.01239-24. Epub 2024 Oct 29. J Virol. 2024. PMID: 39470210 Free PMC article.
-
Cyclophilin A and viral infections.Biochem Biophys Res Commun. 2012 Aug 10;424(4):647-50. doi: 10.1016/j.bbrc.2012.07.024. Epub 2012 Jul 16. Biochem Biophys Res Commun. 2012. PMID: 22814107 Free PMC article. Review.
-
Cyclophilin A as a New Therapeutic Target for Hepatitis C Virus-induced Hepatocellular Carcinoma.Korean J Physiol Pharmacol. 2013 Oct;17(5):375-83. doi: 10.4196/kjpp.2013.17.5.375. Epub 2013 Oct 17. Korean J Physiol Pharmacol. 2013. PMID: 24227937 Free PMC article. Review.
Cited by
-
Identification and characterization of Vibrio vulnificus plpA encoding a phospholipase A2 essential for pathogenesis.J Biol Chem. 2017 Oct 13;292(41):17129-17143. doi: 10.1074/jbc.M117.791657. Epub 2017 Aug 30. J Biol Chem. 2017. PMID: 28855258 Free PMC article.
-
HBV/HIV Coinfection: Impact on the Development and Clinical Treatment of Liver Diseases.Front Med (Lausanne). 2021 Oct 4;8:713981. doi: 10.3389/fmed.2021.713981. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34676223 Free PMC article. Review.
-
Cyclophilin involvement in the replication of hepatitis C virus and other viruses.Biol Chem. 2012 Jul;393(7):579-87. doi: 10.1515/hsz-2012-0151. Biol Chem. 2012. PMID: 22944661 Free PMC article. Review.
-
Inhibition of Cyclophilin A on the replication of red spotted grouper nervous necrosis virus associates with multiple pro-inflammatory factors.Fish Shellfish Immunol. 2019 Sep;92:172-180. doi: 10.1016/j.fsi.2019.05.064. Epub 2019 Jun 5. Fish Shellfish Immunol. 2019. PMID: 31176008 Free PMC article.
-
The role of immunophilins in viral infection.Biochim Biophys Acta. 2015 Oct;1850(10):2103-10. doi: 10.1016/j.bbagen.2014.11.011. Epub 2014 Nov 18. Biochim Biophys Acta. 2015. PMID: 25445708 Free PMC article.
References
-
- Allain, F., C. Vanpouille, M. Carpentier, M. C. Slomianny, S. Durieux, and G. Spik. 2002. Interaction with glycosaminoglycans is required for cyclophilin B to trigger integrin-mediated adhesion of peripheral blood T lymphocytes to extracellular matrix. Proc. Natl. Acad. Sci. U. S. A. 99:2714-2719. - PMC - PubMed
-
- Bonifacino, J. S., and B. S. Glick. 2004. The mechanisms of vesicle budding and fusion. Cell 116:153-166. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

