In-solution virus capture assay helps deconstruct heterogeneous antibody recognition of human immunodeficiency virus type 1
- PMID: 20089658
- PMCID: PMC2838137
- DOI: 10.1128/JVI.02363-09
In-solution virus capture assay helps deconstruct heterogeneous antibody recognition of human immunodeficiency virus type 1
Abstract
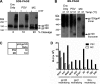
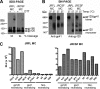
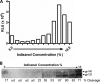
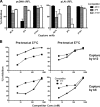
Human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein (Env) on whole virions is heterogeneous, so molecular analysis of Env with monoclonal antibodies (MAbs) is challenging. Virus capture assays (VCAs) involving immobilized MAbs are typically used, but these assays suffer from immobilization artifacts and do not provide binding constants. Furthermore, we show here that certain HIV-1 neutralizing MAbs, including 2G12, 4E10, 2F5, Z13e1, and D5, will capture virion particles completely devoid of Env. We modified the VCA such that MAbs and virions are incubated in solution, and unbound MAbs are removed prior to the capture step. This modification nearly eliminated evidence of Env-independent binding by MAbs to virions and allowed determination of apparent affinity constants in solution. Three important qualitative observations were further revealed. First, neutralizing MAbs 2F5, 4E10, and Z13e1 against the membrane-proximal external region (MPER) of HIV-1 gp41 were found to capture virions efficiently only if a significant amount of uncleaved gp160 or synthetic MPER peptide was present. Second, we show how non-native forms of Env vary by Env genotype and that Env from HIV-1(JR-FL) is more homogeneously trimeric than that from HIV-1(JR-CSF). Third, we determined that Env containing all or parts of gp41, including uncleaved gp160, binds spontaneously to free virions. This exogenous Env is an indiscriminate molecular "bridge" between Env-specific Ab and virions and can affect VCA analyses, particularly using pseudotyped virions. Heterogeneity in Env from endogenous and exogenous sources might also subvert humoral immunity to HIV-1, so in-solution VCAs may help to dissect this heterogeneity for vaccine design purposes.
Figures
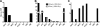
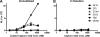
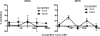
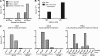
References
-
- Alam, S. M., M. McAdams, D. Boren, M. Rak, R. M. Scearce, F. Gao, Z. T. Camacho, D. Gewirth, G. Kelsoe, P. Chen, and B. F. Haynes. 2007. The role of antibody polyspecificity and lipid reactivity in binding of broadly neutralizing anti-HIV-1 envelope human monoclonal antibodies 2F5 and 4E10 to glycoprotein 41 membrane proximal envelope epitopes. J. Immunol. 178:4424-4435. - PMC - PubMed
-
- Allaway, G. P., K. L. Davis-Bruno, G. A. Beaudry, E. B. Garcia, E. L. Wong, A. M. Ryder, K. W. Hasel, M. C. Gauduin, R. A. Koup, J. S. McDougal, et al. 1995. Expression and characterization of CD4-IgG2, a novel heterotetramer that neutralizes primary HIV type 1 isolates. AIDS Res. Hum. Retrovir. 11:533-539. - PubMed
-
- Astronomo, R. D., H. K. Lee, C. N. Scanlan, R. Pantophlet, C. Y. Huang, I. A. Wilson, O. Blixt, R. A. Dwek, C. H. Wong, and D. R. Burton. 2008. A glycoconjugate antigen based on the recognition motif of a broadly neutralizing human immunodeficiency virus antibody, 2G12, is immunogenic but elicits antibodies unable to bind to the self glycans of gp120. J. Virol. 82:6359-6368. - PMC - PubMed
-
- Bess, J. W., Jr., R. J. Gorelick, W. J. Bosche, L. E. Henderson, and L. O. Arthur. 1997. Microvesicles are a source of contaminating cellular proteins found in purified HIV-1 preparations. Virology 230:134-144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

