HIV-1 Vpr induces the K48-linked polyubiquitination and proteasomal degradation of target cellular proteins to activate ATR and promote G2 arrest
- PMID: 20089662
- PMCID: PMC2838092
- DOI: 10.1128/JVI.02590-09
HIV-1 Vpr induces the K48-linked polyubiquitination and proteasomal degradation of target cellular proteins to activate ATR and promote G2 arrest
Abstract
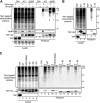
HIV-1 viral protein R (Vpr) induces cell cycle arrest at the G(2)/M phase by a mechanism involving the activation of the DNA damage sensor ATR. We and others recently showed that Vpr performs this function by subverting the activity of the DDB1-CUL4A (VPRBP) E3 ubiquitin ligase. Vpr could thus act as a connector between the E3 ligase and an unknown cellular factor whose ubiquitination would induce G(2) arrest. While attractive, this model is based solely on the indirect observation that some mutants of Vpr retain their interaction with the E3 ligase but fail to induce G(2) arrest. Using a tandem affinity purification approach, we observed that Vpr interacts with ubiquitinated cellular proteins and that this association requires the recruitment of an active E3 ligase given that the depletion of VPRBP by RNA interference or the overexpression of a dominant negative mutant of CUL4A decreased this association. Importantly, G(2)-arrest-defective mutants of Vpr in the C-terminal putative substrate-interacting domain displayed a decreased association with ubiquitinated proteins. We also found that the inhibition of proteasomal activity increased this association and that the ubiquitin chains were at least in part constituted of classical K48 linkages. Interestingly, the inhibition of K48 polyubiquitination specifically impaired the Vpr-induced phosphorylation of H2AX, an early target of ATR, but did not affect UV-induced H2AX phosphorylation. Overall, our results provide direct evidence that the association of Vpr with the DDB1-CUL4A (VPRBP) E3 ubiquitin ligase induces the K48-linked polyubiquitination of as-yet-unknown cellular proteins, resulting in their proteasomal degradation and ultimately leading to the activation of ATR and G(2) arrest.
Figures


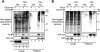



Similar articles
-
Formation of mobile chromatin-associated nuclear foci containing HIV-1 Vpr and VPRBP is critical for the induction of G2 cell cycle arrest.PLoS Pathog. 2010 Sep 2;6(9):e1001080. doi: 10.1371/journal.ppat.1001080. PLoS Pathog. 2010. PMID: 20824083 Free PMC article.
-
HIV-1 Vpr-mediated G2 arrest involves the DDB1-CUL4AVPRBP E3 ubiquitin ligase.PLoS Pathog. 2007 Jul;3(7):e85. doi: 10.1371/journal.ppat.0030085. PLoS Pathog. 2007. PMID: 17630831 Free PMC article.
-
HIV-1 Vpr Protein Enhances Proteasomal Degradation of MCM10 DNA Replication Factor through the Cul4-DDB1[VprBP] E3 Ubiquitin Ligase to Induce G2/M Cell Cycle Arrest.J Biol Chem. 2015 Jul 10;290(28):17380-9. doi: 10.1074/jbc.M115.641522. Epub 2015 Jun 1. J Biol Chem. 2015. PMID: 26032416 Free PMC article.
-
Lentivirus Vpr and Vpx accessory proteins usurp the cullin4-DDB1 (DCAF1) E3 ubiquitin ligase.Curr Opin Virol. 2012 Dec;2(6):755-63. doi: 10.1016/j.coviro.2012.09.010. Epub 2012 Oct 10. Curr Opin Virol. 2012. PMID: 23062609 Free PMC article. Review.
-
The functions of the HIV1 protein Vpr and its action through the DCAF1.DDB1.Cullin4 ubiquitin ligase.Cytokine. 2010 Jul;51(1):1-9. doi: 10.1016/j.cyto.2010.02.018. Epub 2010 Mar 27. Cytokine. 2010. PMID: 20347598 Free PMC article. Review.
Cited by
-
Rapamycin activates autophagy in Hutchinson-Gilford progeria syndrome: implications for normal aging and age-dependent neurodegenerative disorders.Autophagy. 2012 Jan;8(1):147-51. doi: 10.4161/auto.8.1.18331. Epub 2012 Jan 1. Autophagy. 2012. PMID: 22170152 Free PMC article. Review.
-
DNA damage enhances integration of HIV-1 into macrophages by overcoming integrase inhibition.Retrovirology. 2013 Feb 21;10:21. doi: 10.1186/1742-4690-10-21. Retrovirology. 2013. PMID: 23432899 Free PMC article.
-
Trafficking and function of the cystic fibrosis transmembrane conductance regulator: a complex network of posttranslational modifications.Am J Physiol Lung Cell Mol Physiol. 2016 Oct 1;311(4):L719-L733. doi: 10.1152/ajplung.00431.2015. Epub 2016 Jul 29. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 27474090 Free PMC article. Review.
-
HIV-1 Vpr loads uracil DNA glycosylase-2 onto DCAF1, a substrate recognition subunit of a cullin 4A-ring E3 ubiquitin ligase for proteasome-dependent degradation.J Biol Chem. 2010 Nov 26;285(48):37333-41. doi: 10.1074/jbc.M110.133181. Epub 2010 Sep 24. J Biol Chem. 2010. PMID: 20870715 Free PMC article.
-
HIV-1 Vpr redirects host ubiquitination pathway.J Virol. 2014 Aug;88(16):9141-52. doi: 10.1128/JVI.00619-14. Epub 2014 Jun 4. J Virol. 2014. PMID: 24899191 Free PMC article.
References
-
- Andersen, J. L., E. S. Zimmerman, J. L. DeHart, S. Murala, O. Ardon, J. Blackett, J. Chen, and V. Planelles. 2005. ATR and GADD45alpha mediate HIV-1 Vpr-induced apoptosis. Cell Death Differ. 12:326-334. - PubMed
-
- Angers, S., T. Li, X. Yi, M. J. MacCoss, R. T. Moon, and N. Zheng. 2006. Molecular architecture and assembly of the DDB1-CUL4A ubiquitin ligase machinery. Nature 443:590-593. - PubMed
-
- Barry, M., and K. Fruh. 2006. Viral modulators of cullin RING ubiquitin ligases: culling the host defense. Sci. STKE 2006:pe21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

