The direct molecular effects of fatigue and myosin regulatory light chain phosphorylation on the actomyosin contractile apparatus
- PMID: 20089714
- PMCID: PMC2853388
- DOI: 10.1152/ajpregu.00566.2009
The direct molecular effects of fatigue and myosin regulatory light chain phosphorylation on the actomyosin contractile apparatus
Abstract
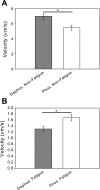
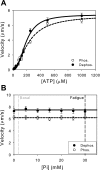
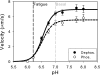
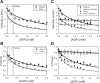
Skeletal muscle, during periods of exertion, experiences several different fatigue-based changes in contractility, including reductions in force, velocity, power output, and energy usage. The fatigue-induced changes in contractility stem from many different factors, including alterations in the levels of metabolites, oxidative damage, and phosphorylation of the myosin regulatory light chain (RLC). Here, we measured the direct molecular effects of fatigue-like conditions on actomyosin's unloaded sliding velocity using the in vitro motility assay. We examined how changes in ATP, ADP, P(i), and pH affect the ability of the myosin to translocate actin and whether the effects of each individual molecular species are additive. We found that the primary causes of the reduction in unloaded sliding velocity are increased [ADP] and lowered pH and that the combined effects of the molecular species are nonadditive. Furthermore, since an increase in RLC phosphorylation is often associated with fatigue, we examined the differential effects of myosin RLC phosphorylation and fatigue on actin filament velocity. We found that phosphorylation of the RLC causes a 22% depression in sliding velocity. On the other hand, RLC phosphorylation ameliorates the slowing of velocity under fatigue-like conditions. We also found that phosphorylation of the myosin RLC increases actomyosin affinity for ADP, suggesting a kinetic role for RLC phosphorylation. Furthermore, we showed that ADP binding to skeletal muscle is load dependent, consistent with the existence of a load-dependent isomerization of the ADP bound state.
Figures

References
-
- Bergstrom M, Hultman E. Energy cost and fatigue during intermittent electrical stimulation of human skeletal muscle. J Appl Physiol 65: 1500–1505, 1988 - PubMed
-
- Butler TM, Siegman MJ, Mooers SU, Barsotti RJ. Myosin light chain phosphorylation does not modulate cross-bridge cycling rate in mouse skeletal muscle. Science 220: 1167–1169, 1983 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

