Melanocortins induce interleukin 6 gene expression and secretion through melanocortin receptors 2 and 5 in 3T3-L1 adipocytes
- PMID: 20089716
- PMCID: PMC3058511
- DOI: 10.1677/JME-09-0161
Melanocortins induce interleukin 6 gene expression and secretion through melanocortin receptors 2 and 5 in 3T3-L1 adipocytes
Abstract
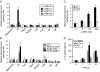
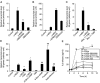
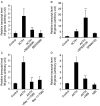
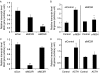
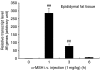
Interleukin 6 (IL6) is a pleiotropic cytokine that not only affects the immune system, but also plays an active role in many physiological events in various organs. Notably, 35% of systemic IL6 originates from adipose tissues under noninflammatory conditions. Here, we describe a previously unknown function of melanocortins in regulating Il6 gene expression and production in 3T3-L1 adipocytes through membrane receptors which are called melanocortin receptors (MCRs). Of the five MCRs that have been cloned, MC2R and MC5R are expressed during adipocyte differentiation. alpha-Melanocyte-stimulating hormone (alpha-MSH) or ACTH treatment of 3T3-L1 adipocytes induces Il6 gene expression and production in a time- and concentration-dependent manner via various signaling pathways including the protein kinase A, p38 mitogen-activated protein kinase, cJun N-terminal kinase, and IkappaB kinase pathways. Specific inhibition of MC2R and MC5R expression with short interfering Mc2r and Mc5r RNAs significantly attenuated the alpha-MSH-induced increase of intracellular cAMP and both the level of Il6 mRNA and secretion of IL6 in 3T3-L1 adipocytes. Finally, when injected into mouse tail vein, alpha-MSH dramatically increased the Il6 transcript levels in epididymal fat pads. These results suggest that alpha-MSH in addition to ACTH may function as a regulator of inflammation by regulating cytokine production.
Figures


Similar articles
-
Characterization of murine melanocortin receptors mediating adipocyte lipolysis and examination of signalling pathways involved.Mol Cell Endocrinol. 2011 Jul 20;341(1-2):9-17. doi: 10.1016/j.mce.2011.03.010. Epub 2011 May 17. Mol Cell Endocrinol. 2011. PMID: 21616121
-
Alpha-MSH signalling via melanocortin 5 receptor promotes lipolysis and impairs re-esterification in adipocytes.Biochim Biophys Acta. 2013 Jul;1831(7):1267-75. doi: 10.1016/j.bbalip.2013.04.008. Biochim Biophys Acta. 2013. PMID: 24046867
-
Nr4a1 siRNA expression attenuates α-MSH regulated gene expression in 3T3-L1 adipocytes.Mol Endocrinol. 2011 Feb;25(2):291-306. doi: 10.1210/me.2010-0231. Epub 2011 Jan 14. Mol Endocrinol. 2011. PMID: 21239615 Free PMC article.
-
The multifunctional human ocular melanocortin system.Prog Retin Eye Res. 2023 Jul;95:101187. doi: 10.1016/j.preteyeres.2023.101187. Epub 2023 May 20. Prog Retin Eye Res. 2023. PMID: 37217094 Review.
-
60 YEARS OF POMC: Melanocortin receptors: evolution of ligand selectivity for melanocortin peptides.J Mol Endocrinol. 2016 May;56(4):T119-33. doi: 10.1530/JME-15-0292. Epub 2016 Jan 20. J Mol Endocrinol. 2016. PMID: 26792827 Review.
Cited by
-
Long Term Osmotic Mini Pump Treatment with Alpha-MSH Improves Myocardial Function in Zucker Diabetic Fatty Rats.Molecules. 2017 Oct 12;22(10):1702. doi: 10.3390/molecules22101702. Molecules. 2017. PMID: 29023410 Free PMC article.
-
Pharmacological Melanocortin 5 Receptor Activation Attenuates Glomerular Injury and Proteinuria in Rats With Puromycin Aminonucleoside Nephrosis.Front Physiol. 2022 Jun 1;13:887641. doi: 10.3389/fphys.2022.887641. eCollection 2022. Front Physiol. 2022. PMID: 35721571 Free PMC article.
-
Exacerbation of autoimmune uveitis by obesity occurs through the melanocortin 5 receptor.J Leukoc Biol. 2019 Oct;106(4):879-887. doi: 10.1002/JLB.MA0119-030RR. Epub 2019 Jul 9. J Leukoc Biol. 2019. PMID: 31287586 Free PMC article.
-
Development of melanoma-targeted polymer micelles by conjugation of a melanocortin 1 receptor (MC1R) specific ligand.J Med Chem. 2011 Dec 8;54(23):8078-84. doi: 10.1021/jm201226w. Epub 2011 Nov 10. J Med Chem. 2011. PMID: 22011200 Free PMC article.
-
Atypical pituitary hormone-target tissue axis.Front Med. 2023 Feb;17(1):1-17. doi: 10.1007/s11684-022-0973-7. Epub 2023 Feb 27. Front Med. 2023. PMID: 36849623 Review.
References
-
- Ajuwon KM, Spurlock ME. Palmitate activates the NF-κB transcription factor and induces IL-6 and TNFalpha expression in 3T3-L1 adipocytes. Journal of Nutrition. 2005;135:1841–1846. - PubMed
-
- An J, Sun Y, Sun R, Rettig MB. Kaposi's sarcoma-associated herpesvirus encoded vFLIP induces cellular IL-6 expression: the role of the NF-κB and JNK/AP1 pathways. Oncogene. 2003;22:3371–3385. - PubMed
-
- An JJ, Rhee Y, Kim SH, Kim DM, Han DH, Hwang JH, Jin YJ, Cha BS, Baik JH, Lee WT, et al. Peripheral effect of alpha-melanocyte-stimulating hormone on fatty acid oxidation in skeletal muscle. Journal of Biological Chemistry. 2007;282:2862–2870. - PubMed
-
- Antunes TT, Gagnon A, Chen B, Pacini F, Smith TJ, Sorisky A. Interleukin-6 release from human abdominal adipose cells is regulated by thyroid-stimulating hormone: effect of adipocyte differentiation and anatomic depot. American Journal of Physiology. Endocrinology and Metabolism. 2006;290:E1140–E1144. - PubMed
-
- Antunes TT, Gagnon A, Langille ML, Sorisky A. Thyroid-stimulating hormone induces interleukin-6 release from human adipocytes through activation of the nuclear factor-κB pathway. Endocrinology. 2008;149:3062–3066. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

