Influenza virus inactivation for studies of antigenicity and phenotypic neuraminidase inhibitor resistance profiling
- PMID: 20089763
- PMCID: PMC2832438
- DOI: 10.1128/JCM.02045-09
Influenza virus inactivation for studies of antigenicity and phenotypic neuraminidase inhibitor resistance profiling
Abstract
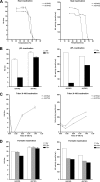
Introduction of a new influenza virus in humans urges quick analysis of its virological and immunological characteristics to determine the impact on public health and to develop protective measures for the human population. At present, however, the necessity of executing pandemic influenza virus research under biosafety level 3 (BSL-3) high-containment conditions severely hampers timely characterization of such viruses. We tested heat, formalin, Triton X-100, and beta-propiolactone treatments for their potencies in inactivating human influenza A(H3N2) and avian A(H7N3) viruses, as well as seasonal and pandemic A(H1N1) virus isolates, while allowing the specimens to retain their virological and immunological properties. Successful heat inactivation coincided with the loss of hemagglutinin (HA) and neuraminidase (NA) characteristics, and beta-propiolactone inactivation reduced the hemagglutination titer and NA activity of the human influenza virus 10-fold or more. Although Triton X-100 treatment resulted in inconsistent HA activity, the NA activities in culture supernatants were enhanced consistently. Nonetheless, formalin treatment permitted the best retention of HA and NA properties. Triton X-100 treatment proved to be the easiest-to-use influenza virus inactivation protocol for application in combination with phenotypic NA inhibitor susceptibility assays, while formalin treatment preserved B-cell and T-cell epitope antigenicity, allowing the detection of both humoral and cellular immune responses. In conclusion, we demonstrated successful influenza virus characterization using formalin- and Triton X-100-inactivated virus samples. Application of these inactivation protocols limits work under BSL-3 conditions to virus culture, thus enabling more timely determination of public health impact and development of protective measures when a new influenza virus, e.g., pandemic A(H1N1)v virus, is introduced in humans.
Figures



Similar articles
-
Evaluation of different inactivation methods for high and low pathogenic avian influenza viruses in egg-fluids for antigen preparation.J Virol Methods. 2015 Sep 15;222:28-33. doi: 10.1016/j.jviromet.2015.05.004. Epub 2015 May 18. J Virol Methods. 2015. PMID: 25997377
-
Neuraminidase inhibitor susceptibility and neuraminidase enzyme kinetics of human influenza A and B viruses circulating in Thailand in 2010-2015.PLoS One. 2018 Jan 11;13(1):e0190877. doi: 10.1371/journal.pone.0190877. eCollection 2018. PLoS One. 2018. PMID: 29324781 Free PMC article.
-
Changes in in vitro susceptibility of influenza A H3N2 viruses to a neuraminidase inhibitor drug during evolution in the human host.J Antimicrob Chemother. 2004 May;53(5):759-65. doi: 10.1093/jac/dkh155. Epub 2004 Mar 17. J Antimicrob Chemother. 2004. PMID: 15028666
-
Monitoring of viral susceptibility: new challenges with the development of influenza NA inhibitors.Rev Med Virol. 2000 Jan-Feb;10(1):45-55. doi: 10.1002/(sici)1099-1654(200001/02)10:1<45::aid-rmv265>3.0.co;2-r. Rev Med Virol. 2000. PMID: 10654004 Review.
-
Neuraminidase inhibitor resistance in influenza viruses and laboratory testing methods.Antivir Ther. 2012;17(1 Pt B):159-73. doi: 10.3851/IMP2067. Epub 2012 Feb 3. Antivir Ther. 2012. PMID: 22311680 Review.
Cited by
-
Intranasal Treatment of Ferrets with Inert Bacterial Spores Reduces Disease Caused by a Challenging H7N9 Avian Influenza Virus.Vaccines (Basel). 2022 Sep 19;10(9):1559. doi: 10.3390/vaccines10091559. Vaccines (Basel). 2022. PMID: 36146637 Free PMC article.
-
Influenza A virus induces interleukin-27 through cyclooxygenase-2 and protein kinase A signaling.J Biol Chem. 2012 Apr 6;287(15):11899-910. doi: 10.1074/jbc.M111.308064. Epub 2012 Feb 16. J Biol Chem. 2012. PMID: 22343630 Free PMC article.
-
Use of lentiviral pseudotypes as an alternative to reassortant or Triton X-100-treated wild-type Influenza viruses in the neuraminidase inhibition enzyme-linked lectin assay.Influenza Other Respir Viruses. 2019 Sep;13(5):504-516. doi: 10.1111/irv.12669. Influenza Other Respir Viruses. 2019. PMID: 31411006 Free PMC article.
-
Tethered Bilayer Lipid Membrane Platform for Screening Triton X-100 Detergent Replacements by Electrochemical Impedance Spectroscopy.Nanomaterials (Basel). 2023 Feb 26;13(5):874. doi: 10.3390/nano13050874. Nanomaterials (Basel). 2023. PMID: 36903751 Free PMC article.
-
An infected chicken kidney cell co-culture ELISpot for enhanced detection of T cell responses to avian influenza and vaccination.J Immunol Methods. 2015 Jan;416:40-8. doi: 10.1016/j.jim.2014.10.012. Epub 2014 Nov 7. J Immunol Methods. 2015. PMID: 25450002 Free PMC article.
References
-
- Bardiya, N., and J. H. Bae. 2005. Influenza vaccines: recent advances in production technologies. Appl. Microbiol. Biotechnol. 67:299-305. - PubMed
-
- Claas, E. C., A. D. Osterhaus, R. van Beek, J. C. De Jong, G. F. Rimmelzwaan, D. A. Senne, S. Krauss, K. F. Shortridge, and R. G. Webster. 1998. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 351:472-477. - PubMed
-
- De Benedictis, P., M. S. Beato, and I. Capua. 2007. Inactivation of avian influenza viruses by chemical agents and physical conditions: a review. Zoonoses Public Health 54:51-68. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

