Regulation of plant glycine decarboxylase by s-nitrosylation and glutathionylation
- PMID: 20089767
- PMCID: PMC2832280
- DOI: 10.1104/pp.109.152579
Regulation of plant glycine decarboxylase by s-nitrosylation and glutathionylation
Abstract
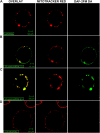
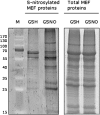
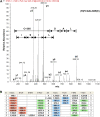
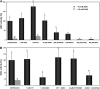
Mitochondria play an essential role in nitric oxide (NO) signal transduction in plants. Using the biotin-switch method in conjunction with nano-liquid chromatography and mass spectrometry, we identified 11 candidate proteins that were S-nitrosylated and/or glutathionylated in mitochondria of Arabidopsis (Arabidopsis thaliana) leaves. These included glycine decarboxylase complex (GDC), a key enzyme of the photorespiratory C(2) cycle in C3 plants. GDC activity was inhibited by S-nitrosoglutathione due to S-nitrosylation/S-glutathionylation of several cysteine residues. Gas-exchange measurements demonstrated that the bacterial elicitor harpin, a strong inducer of reactive oxygen species and NO, inhibits GDC activity. Furthermore, an inhibitor of GDC, aminoacetonitrile, was able to mimic mitochondrial depolarization, hydrogen peroxide production, and cell death in response to stress or harpin treatment of cultured Arabidopsis cells. These findings indicate that the mitochondrial photorespiratory system is involved in the regulation of NO signal transduction in Arabidopsis.
Figures

Similar articles
-
Glycine decarboxylase in C3, C4 and C3-C4 intermediate species.Curr Opin Plant Biol. 2016 Jun;31:29-35. doi: 10.1016/j.pbi.2016.03.011. Epub 2016 Mar 31. Curr Opin Plant Biol. 2016. PMID: 27038285 Review.
-
Proteomic identification of S-nitrosylated proteins in Arabidopsis.Plant Physiol. 2005 Mar;137(3):921-30. doi: 10.1104/pp.104.058719. Epub 2005 Feb 25. Plant Physiol. 2005. PMID: 15734904 Free PMC article.
-
Stoichiometry of two plant glycine decarboxylase complexes and comparison with a cyanobacterial glycine cleavage system.Plant J. 2020 Jul;103(2):801-813. doi: 10.1111/tpj.14773. Epub 2020 May 12. Plant J. 2020. PMID: 32311173
-
Arabidopsis A BOUT DE SOUFFLE is a putative mitochondrial transporter involved in photorespiratory metabolism and is required for meristem growth at ambient CO₂ levels.Plant J. 2013 Mar;73(5):836-49. doi: 10.1111/tpj.12082. Epub 2013 Feb 12. Plant J. 2013. PMID: 23181524
-
Crosstalk between reactive oxygen species and nitric oxide in plants: Key role of S-nitrosoglutathione reductase.Free Radic Biol Med. 2018 Jul;122:110-115. doi: 10.1016/j.freeradbiomed.2017.11.027. Epub 2017 Dec 2. Free Radic Biol Med. 2018. PMID: 29203326 Review.
Cited by
-
Nitric oxide-dependent posttranslational modification in plants: an update.Int J Mol Sci. 2012 Nov 16;13(11):15193-208. doi: 10.3390/ijms131115193. Int J Mol Sci. 2012. PMID: 23203119 Free PMC article. Review.
-
Nitric oxide (NO) modulates low temperature-stress signaling via S-nitrosation, a NO PTM, inducing ethylene biosynthesis inhibition leading to enhanced post-harvest shelf-life of agricultural produce.Physiol Mol Biol Plants. 2023 Dec;29(12):2051-2065. doi: 10.1007/s12298-023-01371-z. Epub 2023 Nov 2. Physiol Mol Biol Plants. 2023. PMID: 38222283 Free PMC article. Review.
-
Alternative oxidase: a mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants.Int J Mol Sci. 2013 Mar 26;14(4):6805-47. doi: 10.3390/ijms14046805. Int J Mol Sci. 2013. PMID: 23531539 Free PMC article. Review.
-
Moving nitrogen to the centre of plant defence against pathogens.Ann Bot. 2017 Mar 1;119(5):703-709. doi: 10.1093/aob/mcw179. Ann Bot. 2017. PMID: 27594647 Free PMC article. Review.
-
S-Nitrosoglutathione Reductase Contributes to Thermotolerance by Modulating High Temperature-Induced Apoplastic H2O2 in Solanum lycopersicum.Front Plant Sci. 2022 Apr 12;13:862649. doi: 10.3389/fpls.2022.862649. eCollection 2022. Front Plant Sci. 2022. PMID: 35498691 Free PMC article.
References
-
- Abe J, Takahashi M, Ishida M, Lee JD, Berk BC. (1997) c-Src is required for oxidative stress-mediated activation of big mitogen-activated protein kinase 1 (BMK1). J Biol Chem 272: 20389–20394 - PubMed
-
- Barchowsky A, Klei LR, Dudek EJ, Swartz HM, James PE. (1999) Stimulation of reactive oxygen, but not reactive nitrogen species, in vascular endothelial cells exposed to low levels of arsenite. Free Radic Biol Med 27: 1405–1412 - PubMed
-
- Barroso JB, Corpas FJ, Carreras A, Sandalio LM, Valderrama R, Palma JM, Lupianez JA, del Rio LA. (1999) Localization of nitric-oxide synthase in plant peroxisomes. J Biol Chem 274: 36729–36733 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

