Glycolysis and the tricarboxylic acid cycle are linked by alanine aminotransferase during hypoxia induced by waterlogging of Lotus japonicus
- PMID: 20089769
- PMCID: PMC2832266
- DOI: 10.1104/pp.109.150045
Glycolysis and the tricarboxylic acid cycle are linked by alanine aminotransferase during hypoxia induced by waterlogging of Lotus japonicus
Abstract
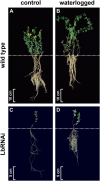
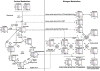
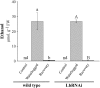
The role of nitrogen metabolism in the survival of prolonged periods of waterlogging was investigated in highly flood-tolerant, nodulated Lotus japonicus plants. Alanine production revealed to be a critical hypoxic pathway. Alanine is the only amino acid whose biosynthesis is not inhibited by nitrogen deficiency resulting from RNA interference silencing of nodular leghemoglobin. The metabolic changes that were induced following waterlogging can be best explained by the activation of alanine metabolism in combination with the modular operation of a split tricarboxylic acid pathway. The sum result of this metabolic scenario is the accumulation of alanine and succinate and the production of extra ATP under hypoxia. The importance of alanine metabolism is discussed with respect to its ability to regulate the level of pyruvate, and this and all other changes are discussed in the context of current models concerning the regulation of plant metabolism.
Figures


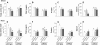



References
-
- Armstrong W, Brändle R, Jackson MB. (1994) Mechanisms of flood tolerance in plants. Acta Bot Neerl 43: 307–358
-
- Bailey-Serres J, Voesenek LACJ. (2008) Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol 59: 313–339 - PubMed
-
- Besson-Bard A, Pugin A, Wendehenne D. (2008) New insights into nitric oxide signaling in plants. Annu Rev Plant Biol 59: 21–39 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

