The heat-inducible transcription factor HsfA2 enhances anoxia tolerance in Arabidopsis
- PMID: 20089772
- PMCID: PMC2832282
- DOI: 10.1104/pp.109.149815
The heat-inducible transcription factor HsfA2 enhances anoxia tolerance in Arabidopsis
Abstract
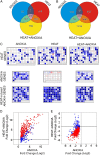
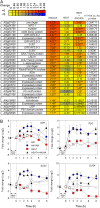
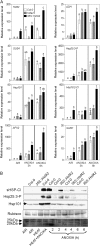
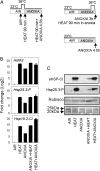
Anoxia induces several heat shock proteins, and a mild heat pretreatment can acclimatize Arabidopsis (Arabidopsis thaliana) seedlings to subsequent anoxic treatment. In this study, we analyzed the response of Arabidopsis seedlings to anoxia, heat, and combined heat + anoxia stress. A significant overlap between the anoxic and the heat responses was observed by whole-genome microarray analysis. Among the transcription factors induced by both heat and anoxia, the heat shock factor A2 (HsfA2), known to be involved in Arabidopsis acclimation to heat and to other abiotic stresses, was strongly induced by anoxia. Heat-dependent acclimation to anoxia is lost in an HsfA2 knockout mutant (hsfa2) as well as in a double mutant for the constitutively expressed HsfA1a/HsfA1b (hsfA1a/1b), indicating that these three heat shock factors cooperate to confer anoxia tolerance. Arabidopsis seedlings that overexpress HsfA2 showed an increased expression of several known targets of this transcription factor and were markedly more tolerant to anoxia as well as to submergence. Anoxia failed to induce HsfA2 target proteins in wild-type seedlings, while overexpression of HsfA2 resulted in the production of HsfA2 targets under anoxia, correlating well with the low anoxia tolerance experiments. These results indicate that there is a considerable overlap between the molecular mechanisms of heat and anoxia tolerance and that HsfA2 is a player in these mechanisms.
Figures



References
-
- Bailey-Serres J, Voesenek LA. (2008) Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol 59: 313–339 - PubMed
-
- Banti V, Loreti E, Novi G, Santaniello A, Alpi A, Perata P. (2008) Heat acclimation and cross-tolerance against anoxia in Arabidopsis. Plant Cell Environ 31: 1029–1037 - PubMed
-
- Bieniawska Z, Barratt DHP, Garlick AP, Thole V, Kruger NJ, Martin C, Zrenner R, Smith AM. (2007) Analysis of the sucrose synthase gene family in Arabidopsis. Plant J 49: 810–828 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

