Nasolacrimal duct closure modulates ocular mucosal and systemic CD4(+) T-cell responses induced following topical ocular or intranasal immunization
- PMID: 20089796
- PMCID: PMC2837957
- DOI: 10.1128/CVI.00347-09
Nasolacrimal duct closure modulates ocular mucosal and systemic CD4(+) T-cell responses induced following topical ocular or intranasal immunization
Abstract
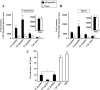
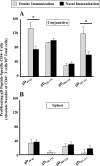
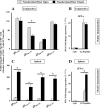
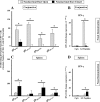
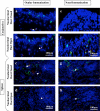
Both topical ocular and topical intranasal immunizations have been reported to stimulate the ocular mucosal immune system (OMIS) and the systemic immune system. Nasolacrimal ducts (NLDs) are the connecting bridges between the OMIS and nasal cavity-associated lymphoid tissue (NALT). These ducts drain topical ocularly administrated solutions into the inferior meatus of the nose to reach the NALT. Inversely, NLDs also drain intranasally administrated solutions to the mucosal surface of the eye and thus the OMIS. This unique anatomical connection between the OMIS and NALT systems provoked us to test whether the OMIS and NALT are immunologically interdependent. In this report, we show that both topical ocular administration and topical intranasal administration of a mixture of immunodominant CD4(+) T-cell epitope peptides from herpes simplex virus type 1 (HSV-1) glycoprotein D (gD) emulsified with the CpG(2007) mucosal adjuvant are capable of inducing local (in conjunctiva) as well as systemic (in spleen) HSV-peptide-specific CD4(+) T-cell responses. Interestingly, surgical closure of NLDs did not significantly alter local ocular mucosal CD4(+) T-cell responses induced following topical ocular immunization but did significantly enhance systemic CD4(+) T-cell responses (as measured by both T-cell proliferation and gamma interferon (IFN-gamma) production; P < 0.005). In contrast, NLD closure significantly decreased ocular mucosal, but not systemic, CD4(+) T-cell responses following intranasal administration of the same vaccine solution (P < 0.001). The study suggests that NALT and the OMIS are immunologically interconnected.
Figures
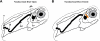


References
-
- BenMohamed, L., Y. Belkaid, E. Loing, K. Brahimi, H. Gras-Masse, and P. Druilhe. 2002. Systemic immune responses induced by mucosal administration of lipopeptides without adjuvant. Eur. J. Immunol. 32:2274-2281. - PubMed
-
- BenMohamed, L., G. Bertrand, C. D. McNamara, H. Gras-Masse, J. Hammer, S. L. Wechsler, and A. B. Nesburn. 2003. Identification of novel immunodominant CD4+ Th1-type T-cell peptide epitopes from herpes simplex virus glycoprotein D that confer protective immunity. J. Virol. 77:9463-9473. - PMC - PubMed
-
- BenMohamed, L., S. L. Wechsler, and A. B. Nesburn. 2002. Lipopeptide vaccines—-yesterday, today, and tomorrow. Lancet Infect. Dis. 2:425-431. - PubMed
-
- Bettahi, I., A. B. Nesburn, S. Yoon, X. Zhang, A. Mohebbi, V. Sue, A. Vanderberg, S. L. Wechsler, and L. BenMohamed. 2007. Protective immunity against ocular herpes infection and disease induced by highly immunogenic self-adjuvanting glycoprotein D lipopeptide vaccines. Invest. Ophthalmol. Vis. Sci. 48:4643-4653. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

