Regionally localized recurrent excitation in the dentate gyrus of a cortical contusion model of posttraumatic epilepsy
- PMID: 20089815
- PMCID: PMC2887624
- DOI: 10.1152/jn.00957.2009
Regionally localized recurrent excitation in the dentate gyrus of a cortical contusion model of posttraumatic epilepsy
Abstract
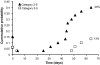
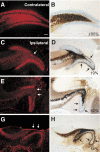
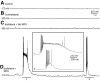
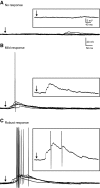
Posttraumatic epilepsy is a frequent consequence of brain trauma, but relatively little is known about how neuronal circuits are chronically altered after closed head injury. We examined whether local recurrent excitatory synaptic connections form between dentate granule cells in mice 8-12 wk after cortical contusion injury. Mice were monitored for behavioral seizures shortly after brain injury and < or = 10 wk postinjury. Injury-induced seizures were observed in 15% of mice, and spontaneous seizures were observed weeks later in 40% of mice. Timm's staining revealed mossy fiber sprouting into the inner molecular layer of the dorsal dentate gyrus ipsilateral to the injury in 95% of mice but not contralateral to the injury or in uninjured controls. Whole cell patch-clamp recordings were made from granule cells in isolated hippocampal brain slices. Cells in slices with posttraumatic mossy fiber sprouting had an increased excitatory postsynaptic current (EPSC) frequency compared with cells in slices without sprouting from injured and control animals (P < 0.001). When perfused with Mg(2+)-free artificial cerebrospinal fluid containing 100 microM picrotoxin, these cells had spontaneous bursts of EPSCs and action potentials. Focal glutamate photostimulation of the granule cell layer evoked a burst of EPSCs and action potentials indicative of recurrent excitatory connections in granule cells of slices with mossy fiber sprouting. In granule cells of slices without sprouting from injured animals and controls, spontaneous or photostimulation-evoked epileptiform activity was never observed. These results suggest that a new regionally localized excitatory network forms between dentate granule cells near the injury site within weeks after cortical contusion head injury.
Figures





Similar articles
-
Recurrent excitatory connectivity in the dentate gyrus of kindled and kainic acid-treated rats.J Neurophysiol. 2000 Feb;83(2):693-704. doi: 10.1152/jn.2000.83.2.693. J Neurophysiol. 2000. PMID: 10669485
-
Recurrent mossy fiber pathway in rat dentate gyrus: synaptic currents evoked in presence and absence of seizure-induced growth.J Neurophysiol. 1999 Apr;81(4):1645-60. doi: 10.1152/jn.1999.81.4.1645. J Neurophysiol. 1999. PMID: 10200201
-
Mossy fiber-granule cell synapses in the normal and epileptic rat dentate gyrus studied with minimal laser photostimulation.J Neurophysiol. 1999 Oct;82(4):1883-94. doi: 10.1152/jn.1999.82.4.1883. J Neurophysiol. 1999. PMID: 10515977
-
Unmasking recurrent excitation generated by mossy fiber sprouting in the epileptic dentate gyrus: an emergent property of a complex system.Prog Brain Res. 2007;163:541-63. doi: 10.1016/S0079-6123(07)63029-5. Prog Brain Res. 2007. PMID: 17765737 Review.
-
Epileptogenesis in the dentate gyrus: a critical perspective.Prog Brain Res. 2007;163:755-73. doi: 10.1016/S0079-6123(07)63041-6. Prog Brain Res. 2007. PMID: 17765749 Review.
Cited by
-
Effects of altered tau expression on dentate granule cell excitability in mice.Exp Neurol. 2021 Sep;343:113766. doi: 10.1016/j.expneurol.2021.113766. Epub 2021 May 21. Exp Neurol. 2021. PMID: 34029610 Free PMC article.
-
LIS1 deficiency promotes dysfunctional synaptic integration of granule cells generated in the developing and adult dentate gyrus.J Neurosci. 2012 Sep 12;32(37):12862-75. doi: 10.1523/JNEUROSCI.1286-12.2012. J Neurosci. 2012. PMID: 22973010 Free PMC article.
-
Adaptive Mossy Cell Circuit Plasticity after Status Epilepticus.J Neurosci. 2022 Apr 6;42(14):3025-3036. doi: 10.1523/JNEUROSCI.1008-21.2022. Epub 2022 Feb 18. J Neurosci. 2022. PMID: 35181595 Free PMC article.
-
Hippocampal granule cell pathology in epilepsy - a possible structural basis for comorbidities of epilepsy?Epilepsy Behav. 2014 Sep;38:105-16. doi: 10.1016/j.yebeh.2013.12.022. Epub 2014 Jan 24. Epilepsy Behav. 2014. PMID: 24468242 Free PMC article. Review.
-
Epilepsy phenotype and its reproducibility after lateral fluid percussion-induced traumatic brain injury in rats: Multicenter EpiBioS4Rx study project 1.Epilepsia. 2024 Feb;65(2):511-526. doi: 10.1111/epi.17838. Epub 2023 Dec 12. Epilepsia. 2024. PMID: 38052475 Free PMC article.
References
-
- Alvarez-Buylla A, Ling CY, Kirn JR. Cresyl violet: a red fluorescent Nissl stain. J Neurosci Methods 33: 129–133, 1990 - PubMed
-
- Annegers JF, Hauser A, Coan SP, Rocca WP. A population-based study of seizures and traumatic brain injuries. N Eng J Med 338: 20–24, 1998 - PubMed
-
- Buckmaster PS, Dudek FE. Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kaintate treated rats. J Comp Neurol 385: 385–404, 1997 - PubMed
-
- Buckmaster PS, Stowbridge BW, Kunkel DD, Schmiege DL, Schwartzkroin PA. Mossy cell axonal projections to the dentate gyrus molecular layer in the rat hippocampal slice. Hippocampus 2: 349–362, 1992 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

