Vav3-deficient mice exhibit a transient delay in cerebellar development
- PMID: 20089829
- PMCID: PMC2836963
- DOI: 10.1091/mbc.e09-04-0292
Vav3-deficient mice exhibit a transient delay in cerebellar development
Abstract
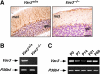
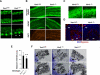
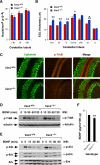
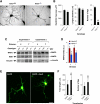
Vav3 is a guanosine diphosphate/guanosine triphosphate exchange factor for Rho/Rac GTPases that has been involved in functions related to the hematopoietic system, bone formation, cardiovascular regulation, angiogenesis, and axon guidance. We report here that Vav3 is expressed at high levels in Purkinje and granule cells, suggesting additional roles for this protein in the cerebellum. Consistent with this hypothesis, we demonstrate using Vav3-deficient mice that this protein contributes to Purkinje cell dendritogenesis, the survival of granule cells of the internal granular layer, the timely migration of granule cells of the external granular layer, and to the formation of the cerebellar intercrural fissure. With the exception of the latter defect, the dysfunctions found in Vav3(-/-) mice only occur at well-defined postnatal developmental stages and disappear, or become ameliorated, in older animals. Vav2-deficient mice do not show any of those defects. Using primary neuronal cultures, we show that Vav3 is important for dendrite branching, but not for primary dendritogenesis, in Purkinje and granule cells. Vav3 function in the cerebellum is functionally relevant, because Vav3(-/-) mice show marked motor coordination and gaiting deficiencies in the postnatal period. These results indicate that Vav3 function contributes to the timely developmental progression of the cerebellum.
Figures



Similar articles
-
Vav3 is involved in GABAergic axon guidance events important for the proper function of brainstem neurons controlling cardiovascular, respiratory, and renal parameters.Mol Biol Cell. 2010 Dec;21(23):4251-63. doi: 10.1091/mbc.E10-07-0639. Epub 2010 Oct 6. Mol Biol Cell. 2010. PMID: 20926682 Free PMC article.
-
Deletion of the Nucleotide Exchange Factor Vav3 Enhances Axonal Complexity and Synapse Formation but Tampers Activity of Hippocampal Neuronal Networks In Vitro.Int J Mol Sci. 2020 Jan 28;21(3):856. doi: 10.3390/ijms21030856. Int J Mol Sci. 2020. PMID: 32013053 Free PMC article.
-
Expression of Rho GTPases Rho-A and Rac1 in the adult and developing gerbil cerebellum.Int J Dev Neurosci. 2008 Nov;26(7):723-32. doi: 10.1016/j.ijdevneu.2008.07.010. Epub 2008 Jul 17. Int J Dev Neurosci. 2008. PMID: 18682288
-
Involvement of the guanine nucleotide exchange factor Vav3 in central nervous system development and plasticity.Biol Chem. 2017 May 1;398(5-6):663-675. doi: 10.1515/hsz-2016-0275. Biol Chem. 2017. PMID: 28214347 Review.
-
Cytodifferentiation of Bergmann glia and its relationship with Purkinje cells.Anat Sci Int. 2002 Jun;77(2):94-108. doi: 10.1046/j.0022-7722.2002.00021.x. Anat Sci Int. 2002. PMID: 12418089 Review.
Cited by
-
Identification of a Vav2-dependent mechanism for GDNF/Ret control of mesolimbic DAT trafficking.Nat Neurosci. 2015 Aug;18(8):1084-93. doi: 10.1038/nn.4060. Epub 2015 Jul 6. Nat Neurosci. 2015. PMID: 26147533
-
Genomic signatures of high-altitude adaptation in Ethiopian sheep populations.Genes Genomics. 2019 Aug;41(8):973-981. doi: 10.1007/s13258-019-00820-y. Epub 2019 May 22. Genes Genomics. 2019. PMID: 31119684
-
Genetic adaptation to high altitude in the Ethiopian highlands.Genome Biol. 2012 Jan 20;13(1):R1. doi: 10.1186/gb-2012-13-1-r1. Genome Biol. 2012. PMID: 22264333 Free PMC article.
-
Lysine Acetylation Reshapes the Downstream Signaling Landscape of Vav1 in Lymphocytes.Cells. 2020 Mar 4;9(3):609. doi: 10.3390/cells9030609. Cells. 2020. PMID: 32143292 Free PMC article.
-
The role of Rho GTPase proteins in CNS neuronal migration.Dev Neurobiol. 2011 Jun;71(6):528-53. doi: 10.1002/dneu.20850. Dev Neurobiol. 2011. PMID: 21557504 Free PMC article. Review.
References
-
- Adams N. C., Tomoda T., Cooper M., Dietz G., Hatten M. E. Mice that lack astrotactin have slowed neuronal migration. Development. 2002;129:965–972. - PubMed
-
- Aghazadeh B., Lowry W. E., Huang X. Y., Rosen M. K. Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell. 2000;102:625–633. - PubMed
-
- Altman J. Postnatal development of the cerebellar cortex in the rat. I. The external germinal layer and the transitional molecular layer. J. Comp. Neurol. 1972a;145:353–397. - PubMed
-
- Altman J. Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer. J. Comp. Neurol. 1972b;145:399–463. - PubMed
-
- Altman J. Postnatal development of the cerebellar cortex in the rat. III. Maturation of the components of the granular layer. J. Comp. Neurol. 1972c;145:465–513. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

