Emi2 inhibition of the anaphase-promoting complex/cyclosome absolutely requires Emi2 binding via the C-terminal RL tail
- PMID: 20089832
- PMCID: PMC2836971
- DOI: 10.1091/mbc.e09-11-0974
Emi2 inhibition of the anaphase-promoting complex/cyclosome absolutely requires Emi2 binding via the C-terminal RL tail
Abstract
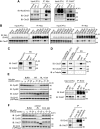
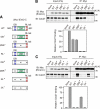
Emi2 (also called Erp1) inhibits the anaphase-promoting complex/cyclosome (APC/C) and thereby causes metaphase II arrest in unfertilized vertebrate eggs. Both the D-box and the zinc-binding region (ZBR) of Emi2 have been implicated in APC/C inhibition. However, it is not well known how Emi2 interacts with and hence inhibits the APC/C. Here we show that Emi2 binds the APC/C via the C-terminal tail, termed here the RL tail. When expressed in Xenopus oocytes and egg extracts, Emi2 lacking the RL tail fails to interact with and inhibit the APC/C. The RL tail itself can directly bind to the APC/C, and, when added to egg extracts, either an excess of RL tail peptides or anti-RL tail peptide antibody can dissociate endogenous Emi2 from the APC/C, thus allowing APC/C activation. Furthermore, and importantly, the RL tail-mediated binding apparently promotes the inhibitory interactions of the D-box and the ZBR (of Emi2) with the APC/C. Finally, Emi1, a somatic paralog of Emi2, also has a functionally similar RL tail. We propose that the RL tail of Emi1/Emi2 serves as a docking site for the APC/C, thereby promoting the interaction and inhibition of the APC/C by the D-box and the ZBR.
Figures

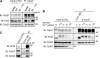


References
-
- Castro A., Bernis C., Vigneron S., Labbe J. C., Lorca T. The anaphase-promoting complex: a key factor in the regulation of cell cycle. Oncogene. 2005;24:314–325. - PubMed
-
- Ellman G. L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. - PubMed
-
- Gross S. D., Schwab M. S., Taieb F. E., Lewellyn A. L., Qian Y. W., Maller J. L. The critical role of the MAP kinase pathway in meiosis II in Xenopus oocytes is mediated by p90Rsk. Curr. Biol. 2000;10:430–438. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

