Transforming growth factor-beta1 induces transdifferentiation of myoblasts into myofibroblasts via up-regulation of sphingosine kinase-1/S1P3 axis
- PMID: 20089836
- PMCID: PMC2836962
- DOI: 10.1091/mbc.e09-09-0812
Transforming growth factor-beta1 induces transdifferentiation of myoblasts into myofibroblasts via up-regulation of sphingosine kinase-1/S1P3 axis
Abstract
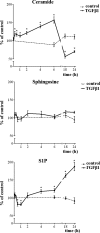
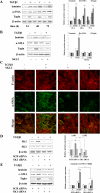
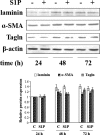
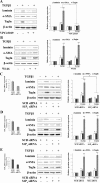
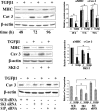
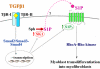
The pleiotropic cytokine transforming growth factor (TGF)-beta1 is a key player in the onset of skeletal muscle fibrosis, which hampers tissue repair. However, the molecular mechanisms implicated in TGFbeta1-dependent transdifferentiation of myoblasts into myofibroblasts are presently unknown. Here, we show that TGFbeta1 up-regulates sphingosine kinase (SK)-1 in C2C12 myoblasts in a Smad-dependent manner, and concomitantly modifies the expression of sphingosine 1-phosphate (S1P) receptors (S1PRs). Notably, pharmacological or short interfering RNA-mediated inhibition of SK1 prevented the induction of fibrotic markers by TGFbeta1. Moreover, inhibition of S1P(3), which became the highest expressed S1PR after TGFbeta1 challenge, strongly attenuated the profibrotic response to TGFbeta1. Furthermore, downstream of S1P(3), Rho/Rho kinase signaling was found critically implicated in the profibrotic action of TGFbeta1. Importantly, we demonstrate that SK/S1P axis, known to play a key role in myogenesis via S1P(2), consequently to TGFbeta1-dependent S1PR pattern remodeling, becomes responsible for transmitting a profibrotic, antidifferentiating action. This study provides new compelling information on the mechanism by which TGFbeta1 gives rise to fibrosis in skeletal muscle, opening new perspectives for its pharmacological treatment. Moreover, it highlights the pleiotropic role of SK/S1P axis in skeletal myoblasts that, depending on the expressed S1PR pattern, seems capable of eliciting multiple, even contrasting biological responses.
Figures





References
-
- Alvarez S. E., Milstien S., Spiegel S. Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol. Metab. 2007;18:300–307. - PubMed
-
- Best T. M., Hunter K. D. Muscle injury and repair. Phys. Med. Rehabil. Clin. N. Am. 2000;11:251–266. - PubMed
-
- Derynck R., Zhang Y. E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. - PubMed
-
- Dolman M. E., Fretz M. M., Segers G. J., Lacombe M., Prakash J., Storm G., Hennink W. E., Kok R. J. Renal targeting of kinase inhibitors. Int. J. Pharm. 2008;364:249–257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

