Mitochondrial dysfunction confers resistance to multiple drugs in Caenorhabditis elegans
- PMID: 20089839
- PMCID: PMC2836976
- DOI: 10.1091/mbc.e09-08-0673
Mitochondrial dysfunction confers resistance to multiple drugs in Caenorhabditis elegans
Abstract
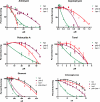
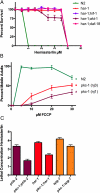
In a previous genetic screen for Caenorhabditis elegans mutants that survive in the presence of an antimitotic drug, hemiasterlin, we identified eight strong mutants. Two of these were found to be resistant to multiple toxins, and in one of these we identified a missense mutation in phb-2, which encodes the mitochondrial protein prohibitin 2. Here we identify two additional mutations that confer drug resistance, spg-7 and har-1, also in genes encoding mitochondrial proteins. Other mitochondrial mutants, isp-1, eat-3, and clk-1, were also found to be drug-resistant. Respiratory complex inhibitors, FCCP and oligomycin, and a producer of reactive oxygen species (ROS), paraquat, all rescued wild-type worms from hemiasterlin toxicity. Worms lacking mitochondrial superoxide dismutase (MnSOD) were modestly drug-resistant, and elimination of MnSOD in the phb-2, har-1, and spg-7 mutants enhanced resistance. The antioxidant N-acetyl-l-cysteine prevented mitochondrial inhibitors from rescuing wild-type worms from hemiasterlin and sensitized mutants to the toxin, suggesting that a mechanism sensitive to ROS is necessary to trigger drug resistance in C. elegans. Using genetics, we show that this drug resistance requires pkc-1, the C. elegans ortholog of human PKCepsilon.
Figures




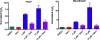

Similar articles
-
A missense mutation in Caenorhabditis elegans prohibitin 2 confers an atypical multidrug resistance.Proc Natl Acad Sci U S A. 2006 Oct 17;103(42):15523-8. doi: 10.1073/pnas.0607338103. Epub 2006 Oct 10. Proc Natl Acad Sci U S A. 2006. PMID: 17032754 Free PMC article.
-
The C. elegans Opa1 homologue EAT-3 is essential for resistance to free radicals.PLoS Genet. 2008 Feb 29;4(2):e1000022. doi: 10.1371/journal.pgen.1000022. PLoS Genet. 2008. PMID: 18454199 Free PMC article.
-
Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans.PLoS Genet. 2009 Feb;5(2):e1000361. doi: 10.1371/journal.pgen.1000361. Epub 2009 Feb 6. PLoS Genet. 2009. PMID: 19197346 Free PMC article.
-
Effects of the mitochondrial respiratory chain on longevity in C. elegans.Exp Gerontol. 2014 Aug;56:245-55. doi: 10.1016/j.exger.2014.03.028. Epub 2014 Apr 5. Exp Gerontol. 2014. PMID: 24709342 Review.
-
Mitochondrial respiration and reactive oxygen species in C. elegans.Exp Gerontol. 2006 Oct;41(10):957-67. doi: 10.1016/j.exger.2006.06.056. Epub 2006 Aug 21. Exp Gerontol. 2006. PMID: 16919906 Review.
Cited by
-
Tryptophan Metabolism in Caenorhabditis elegans Links Aggregation Behavior to Nutritional Status.ACS Chem Biol. 2019 Jan 18;14(1):50-57. doi: 10.1021/acschembio.8b00872. Epub 2018 Dec 26. ACS Chem Biol. 2019. PMID: 30586284 Free PMC article.
-
Respiratory analysis as a tool to detect physiological changes in Anisakis larvae subjected to stress.Parasitol Res. 2019 Apr;118(4):1127-1135. doi: 10.1007/s00436-019-06260-7. Epub 2019 Feb 21. Parasitol Res. 2019. PMID: 30790039
-
Fluorizoline-induced apoptosis requires prohibitins in nematodes and human cells.Apoptosis. 2021 Feb;26(1-2):83-95. doi: 10.1007/s10495-020-01651-z. Epub 2021 Jan 2. Apoptosis. 2021. PMID: 33387147
-
In vitro evolution and whole genome analysis to study chemotherapy drug resistance in haploid human cells.Sci Rep. 2024 Jun 18;14(1):13989. doi: 10.1038/s41598-024-63943-7. Sci Rep. 2024. PMID: 38886371 Free PMC article.
-
Neurodegeneration-associated mitochondrial proteins, CHCHD2 and CHCHD10-what distinguishes the two?Front Cell Dev Biol. 2022 Sep 9;10:996061. doi: 10.3389/fcell.2022.996061. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36158221 Free PMC article. Review.
References
-
- Ayral-Kaloustian S., Zask A. Taltobulin—oncolytic drug tubulin polymerization inhibitor antimitotic drug. Drugs Future. 2005;30:254–260.
-
- Balaban R. S., Nemoto S., Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

