Differential localization and dynamics of class I myosins in the enterocyte microvillus
- PMID: 20089841
- PMCID: PMC2836977
- DOI: 10.1091/mbc.e09-07-0638
Differential localization and dynamics of class I myosins in the enterocyte microvillus
Abstract
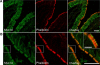
Epithelial cells lining the intestinal tract build an apical array of microvilli known as the brush border. Each microvillus is a cylindrical membrane protrusion that is linked to a supporting actin bundle by myosin-1a (Myo1a). Mice lacking Myo1a demonstrate no overt physiological symptoms, suggesting that other myosins may compensate for the loss of Myo1a in these animals. To investigate changes in the microvillar myosin population that may limit the Myo1a KO phenotype, we performed proteomic analysis on WT and Myo1a KO brush borders. These studies revealed that WT brush borders also contain the short-tailed class I myosin, myosin-1d (Myo1d). Myo1d localizes to the terminal web and striking puncta at the tips of microvilli. In the absence of Myo1a, Myo1d peptide counts increase twofold; this motor also redistributes along the length of microvilli, into compartments normally occupied by Myo1a. FRAP studies demonstrate that Myo1a is less dynamic than Myo1d, providing a mechanistic explanation for the observed differential localization. These data suggest that Myo1d may be the primary compensating class I myosin in the Myo1a KO model; they also suggest that dynamics govern the localization and function of different yet closely related myosins that target common actin structures.
Figures

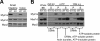



References
-
- Belyantseva I. A., Boger E. T., Naz S., Frolenkov G. I., Sellers J. R., Ahmed Z. M., Griffith A. J., Friedman T. B. Myosin-XVa is required for tip localization of whirlin and differential elongation of hair-cell stereocilia. Nat. Cell Biol. 2005;7:148–156. - PubMed
-
- Berg J. S., Cheney R. E. Myosin-X is an unconventional myosin that undergoes intrafilopodial motility. Nat. Cell Biol. 2002;4:246–250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

