Globular adiponectin as a complete mesoangioblast regulator: role in proliferation, survival, motility, and skeletal muscle differentiation
- PMID: 20089845
- PMCID: PMC2836966
- DOI: 10.1091/mbc.e09-04-0310
Globular adiponectin as a complete mesoangioblast regulator: role in proliferation, survival, motility, and skeletal muscle differentiation
Abstract
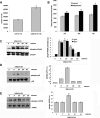
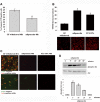
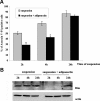
Mesoangioblasts are progenitor endowed with multipotent mesoderm differentiation ability. Despite the promising results obtained with mesoangioblast transplantation in muscle dystrophy, an improvement of their efficient engrafting and survival within damaged muscles, as well as their ex vivo activation/expansion and commitment toward myogenic lineage, is highly needed and should greatly increase their therapeutic potential. We show that globular adiponectin, an adipokine endowed with metabolic and differentiating functions for muscles, regulates vital cues of mesoangioblast cell biology. The adipokine drives mesoangioblasts to entry cell cycle and strongly counteracts the apoptotic process triggered by growth factor withdrawal, thereby serving as an activating and prosurvival stem cell factor. In addition, adiponectin provides a specific protection against anoikis, the apoptotic death due to lack of anchorage to extracellular matrix, suggesting a key protective role for these nonresident stem cells after systemic injection. Finally, adiponectin behaves as a chemoattractive factor toward mature myotubes and stimulates their differentiation toward the skeletal muscle lineage, serving as a positive regulator in mesoangioblast homing to injured or diseased muscles. We conclude that adiponectin exerts several advantageous effects on mesoangioblasts, potentially valuable to improve their efficacy in cell based therapies of diseased muscles.
Figures
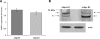
Similar articles
-
Ex vivo treatment with nitric oxide increases mesoangioblast therapeutic efficacy in muscular dystrophy.J Cell Sci. 2006 Dec 15;119(Pt 24):5114-23. doi: 10.1242/jcs.03300. J Cell Sci. 2006. PMID: 17158915
-
Oxidative stress preconditioning of mouse perivascular myogenic progenitors selects a subpopulation of cells with a distinct survival advantage in vitro and in vivo.Cell Death Dis. 2018 Jan 3;9(1):1. doi: 10.1038/s41419-017-0012-9. Cell Death Dis. 2018. PMID: 29298988 Free PMC article.
-
Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation.J Cell Biol. 2004 Feb 2;164(3):441-9. doi: 10.1083/jcb.200304135. Epub 2004 Jan 26. J Cell Biol. 2004. PMID: 14744997 Free PMC article.
-
Mesoangioblasts of inclusion-body myositis: a twofold tool to study pathogenic mechanisms and enhance defective muscle regeneration.Acta Myol. 2011 Jun;30(1):24-8. Acta Myol. 2011. PMID: 21842589 Free PMC article. Review.
-
Adiponectin as a tissue regenerating hormone: more than a metabolic function.Cell Mol Life Sci. 2014 May;71(10):1917-25. doi: 10.1007/s00018-013-1537-4. Epub 2013 Dec 10. Cell Mol Life Sci. 2014. PMID: 24322911 Free PMC article. Review.
Cited by
-
Up-regulation of adiponectin expression in antigravitational soleus muscle in response to unloading followed by reloading, and functional overloading in mice.PLoS One. 2013 Dec 6;8(12):e81929. doi: 10.1371/journal.pone.0081929. eCollection 2013. PLoS One. 2013. PMID: 24324732 Free PMC article.
-
Recent advances and future avenues in understanding the role of adipose tissue cross talk in mediating skeletal muscle mass and function with ageing.Geroscience. 2021 Feb;43(1):85-110. doi: 10.1007/s11357-021-00322-4. Epub 2021 Feb 2. Geroscience. 2021. PMID: 33528828 Free PMC article. Review.
-
Adiponectin Is a Contributing Factor of Low Appendicular Lean Mass in Older Community-Dwelling Women: A Cross-Sectional Study.J Clin Med. 2022 Dec 2;11(23):7175. doi: 10.3390/jcm11237175. J Clin Med. 2022. PMID: 36498747 Free PMC article.
-
Adiponectin pretreatment counteracts the detrimental effect of a diabetic environment on endothelial progenitors.Diabetes. 2011 Feb;60(2):652-61. doi: 10.2337/db10-0240. Diabetes. 2011. PMID: 21270275 Free PMC article.
-
Adiponectin protects endothelial cells from the damages induced by the intermittent high level of glucose.Endocrine. 2011 Dec;40(3):386-93. doi: 10.1007/s12020-011-9531-9. Epub 2011 Sep 24. Endocrine. 2011. PMID: 21948177
References
-
- Ando Y., Inaba M., Sakaguchi Y., Tsuda M., Quan G. K., Omae M., Okazaki K., Ikehara S. Subcutaneous adipose tissue-derived stem cells facilitate colonic mucosal recovery from 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis in rats. Inflamm. Bowel Dis. 2008;14:826–838. - PubMed
-
- Berg A. H., Combs T. P., Scherer P. E. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol. Metab. 2002;13:84–89. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

