Chromogranin B gene ablation reduces the catecholamine cargo and decelerates exocytosis in chromaffin secretory vesicles
- PMID: 20089903
- PMCID: PMC6633114
- DOI: 10.1523/JNEUROSCI.2894-09.2010
Chromogranin B gene ablation reduces the catecholamine cargo and decelerates exocytosis in chromaffin secretory vesicles
Abstract
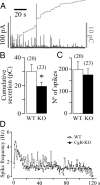
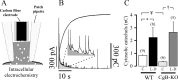
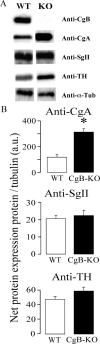
Chromogranins/secretogranins (Cgs) are the major soluble proteins of large dense-core secretory vesicles (LDCVs). We have recently reported that the absence of chromogranin A (CgA) caused important changes in the accumulation and in the exocytosis of catecholamines (CAs) using a CgA-knock-out (CgA-KO) mouse. Here, we have analyzed a CgB-KO mouse strain that can be maintained in homozygosis. These mice have 36% less adrenomedullary epinephrine when compared to Chgb(+/+) [wild type (WT)], whereas the norepinephrine content was similar. The total evoked release of CA was 33% lower than WT mice. This decrease was not due to a lower frequency of exocytotic events but to less secretion per quantum (approximately 30%) measured by amperometry; amperometric spikes exhibited a slower ascending but a normal decaying phase. Cell incubation with L-DOPA increased the vesicle CA content of WT but not of the CgB-KO cells. Intracellular electrochemistry, using patch amperometry, showed that L-DOPA overload produced a significantly larger increase in cytosolic CAs in cells from the KO animals than chromaffin cells from the WT. These data indicate that the mechanisms for vesicular accumulation of CAs in the CgB-KO cells were saturated, while there was ample capacity for further accumulation in WT cells. Protein analysis of LDCVs showed the overexpression of CgA as well as other proteins apparently unrelated to the secretory process. We conclude that CgB, like CgA, is a highly efficient system directly involved in monoamine accumulation and in the kinetics of exocytosis from LDCVs.
Figures


Similar articles
-
The crucial role of chromogranins in storage and exocytosis revealed using chromaffin cells from chromogranin A null mouse.J Neurosci. 2008 Mar 26;28(13):3350-8. doi: 10.1523/JNEUROSCI.5292-07.2008. J Neurosci. 2008. PMID: 18367602 Free PMC article.
-
The functional role of chromogranins in exocytosis.J Mol Neurosci. 2012 Oct;48(2):317-22. doi: 10.1007/s12031-012-9736-2. Epub 2012 Mar 14. J Mol Neurosci. 2012. PMID: 22415354
-
Chromogranins as regulators of exocytosis.J Neurochem. 2010 Jul;114(2):335-43. doi: 10.1111/j.1471-4159.2010.06786.x. Epub 2010 Apr 29. J Neurochem. 2010. PMID: 20456013 Review.
-
Impact of Chromogranin A deficiency on catecholamine storage, catecholamine granule morphology and chromaffin cell energy metabolism in vivo.Cell Tissue Res. 2016 Mar;363(3):693-712. doi: 10.1007/s00441-015-2316-3. Epub 2015 Nov 16. Cell Tissue Res. 2016. PMID: 26572539 Free PMC article.
-
Chromogranins A and B as regulators of vesicle cargo and exocytosis.Cell Mol Neurobiol. 2010 Nov;30(8):1181-7. doi: 10.1007/s10571-010-9584-y. Epub 2010 Nov 3. Cell Mol Neurobiol. 2010. PMID: 21046455 Free PMC article. Review.
Cited by
-
Isolation and Purification of Chromaffin Granules from Adrenal Glands and Cultured Neuroendocrine Cells.Methods Mol Biol. 2023;2565:283-296. doi: 10.1007/978-1-0716-2671-9_19. Methods Mol Biol. 2023. PMID: 36205901
-
Intravesicular factors controlling exocytosis in chromaffin cells.Cell Mol Neurobiol. 2010 Nov;30(8):1359-64. doi: 10.1007/s10571-010-9589-6. Epub 2010 Nov 3. Cell Mol Neurobiol. 2010. PMID: 21046452 Free PMC article.
-
ATP: The crucial component of secretory vesicles.Proc Natl Acad Sci U S A. 2016 Jul 12;113(28):E4098-106. doi: 10.1073/pnas.1600690113. Epub 2016 Jun 24. Proc Natl Acad Sci U S A. 2016. PMID: 27342860 Free PMC article.
-
The extended granin family: structure, function, and biomedical implications.Endocr Rev. 2011 Dec;32(6):755-97. doi: 10.1210/er.2010-0027. Epub 2011 Aug 23. Endocr Rev. 2011. PMID: 21862681 Free PMC article. Review.
-
Mice overexpressing chromogranin A display hypergranulogenic adrenal glands with attenuated ATP levels contributing to the hypertensive phenotype.J Hypertens. 2018 May;36(5):1115-1128. doi: 10.1097/HJH.0000000000001678. J Hypertens. 2018. PMID: 29389743 Free PMC article.
References
-
- Apps DK. Membrane and soluble proteins of adrenal chromaffin granules. Semin Cell Dev Biol. 1997;8:121–131. - PubMed
-
- Borges R, Sala F, Garcia AG. Continuous monitoring of catecholamine release from perfused cat adrenals. J Neurosci Methods. 1986;16:289–300. - PubMed
-
- Colliver TL, Hess EJ, Ewing AG. Amperometric analysis of exocytosis at chromaffin cells from genetically distinct mice. J Neurosci Methods. 2001;105:95–103. - PubMed
-
- Dernick G, Gong LW, Tabares L, Alvarez de Toledo G, Lindau M. Patch amperometry: high-resolution measurements of single-vesicle fusion and release. Nat Methods. 2005;2:699–708. - PubMed
-
- Gerdes HH, Glombik MM. Signal-mediated sorting to the regulated pathway of protein secretion. Ann Anat. 1999;181:447–453. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
