Genetically defined inhibitory neurons in the mouse spinal cord dorsal horn: a possible source of rhythmic inhibition of motoneurons during fictive locomotion
- PMID: 20089922
- PMCID: PMC5061569
- DOI: 10.1523/JNEUROSCI.1401-09.2010
Genetically defined inhibitory neurons in the mouse spinal cord dorsal horn: a possible source of rhythmic inhibition of motoneurons during fictive locomotion
Abstract
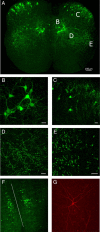
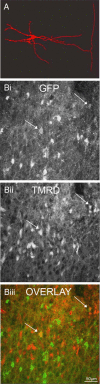
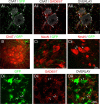
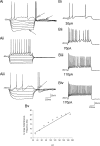
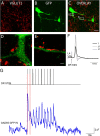
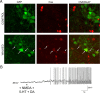
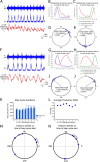
To ensure alternation of flexor and extensor muscles during locomotion, the spinal locomotor network provides rhythmic inhibition to motoneurons. The source of this inhibition in mammals is incompletely defined. We have identified a population of GABAergic interneurons located in medial laminae V/VI that express green fluorescent protein (GFP) in glutamic acid decarboxylase-65::GFP transgenic mice. Immunohistochemical studies revealed GFP+ terminals in apposition to motoneuronal somata, neurons in Clarke's column, and in laminae V/VI where they apposed GFP+ interneurons, thus forming putative reciprocal connections. Whole-cell patch-clamp recordings from GFP+ interneurons in spinal cord slices revealed a range of electrophysiological profiles, including sag and postinhibitory rebound potentials. Most neurons fired tonically in response to depolarizing current injection. Calcium transients demonstrated by two-photon excitation microscopy in the hemisected spinal cord were recorded in response to low-threshold dorsal root stimulation, indicating these neurons receive primary afferent input. Following a locomotor task, the number of GFP+ neurons expressing Fos increased, indicating that these neurons are active during locomotion. During fictive locomotion induced in the hemisected spinal cord, two-photon excitation imaging demonstrated rhythmic calcium activity in these interneurons, which correlated with the termination of ventral root bursts. We suggest that these dorsomedial GABAergic interneurons are involved in spinal locomotor networks, and may provide direct rhythmic inhibitory input to motoneurons during locomotion.
Figures
Similar articles
-
Locomotor-related activity of GABAergic interneurons localized in the ventrolateral region in the isolated spinal cord of neonatal mice.J Neurophysiol. 2011 Oct;106(4):1782-92. doi: 10.1152/jn.00385.2011. Epub 2011 Jul 6. J Neurophysiol. 2011. PMID: 21734105
-
Activity of Renshaw cells during locomotor-like rhythmic activity in the isolated spinal cord of neonatal mice.J Neurosci. 2006 May 17;26(20):5320-8. doi: 10.1523/JNEUROSCI.5127-05.2006. J Neurosci. 2006. PMID: 16707784 Free PMC article.
-
Low-threshold primary afferent drive onto GABAergic interneurons in the superficial dorsal horn of the mouse.J Neurosci. 2009 Jan 21;29(3):686-95. doi: 10.1523/JNEUROSCI.5120-08.2009. J Neurosci. 2009. PMID: 19158295 Free PMC article.
-
Development of spinal motor networks in the chick embryo.J Exp Zool. 1992 Mar 1;261(3):261-73. doi: 10.1002/jez.1402610306. J Exp Zool. 1992. PMID: 1629659 Review.
-
The neuronal network for locomotion in the lamprey spinal cord: evidence for the involvement of commissural interneurons.J Physiol Paris. 1995;89(4-6):221-33. doi: 10.1016/0928-4257(96)83638-2. J Physiol Paris. 1995. PMID: 8861820 Review.
Cited by
-
Functional Characterization of Lamina X Neurons in ex-Vivo Spinal Cord Preparation.Front Cell Neurosci. 2017 Nov 1;11:342. doi: 10.3389/fncel.2017.00342. eCollection 2017. Front Cell Neurosci. 2017. PMID: 29163053 Free PMC article.
-
Requirement of neuronal connexin36 in pathways mediating presynaptic inhibition of primary afferents in functionally mature mouse spinal cord.J Physiol. 2012 Aug 15;590(16):3821-39. doi: 10.1113/jphysiol.2011.225987. Epub 2012 May 21. J Physiol. 2012. PMID: 22615430 Free PMC article.
-
Modern Brain Mapping - What Do We Map Nowadays?Front Psychiatry. 2015 Jun 16;6:89. doi: 10.3389/fpsyt.2015.00089. eCollection 2015. Front Psychiatry. 2015. PMID: 26136692 Free PMC article. Review. No abstract available.
-
The use of PRV-Bartha to define premotor inputs to lumbar motoneurons in the neonatal spinal cord of the mouse.PLoS One. 2010 Jul 23;5(7):e11743. doi: 10.1371/journal.pone.0011743. PLoS One. 2010. PMID: 20668534 Free PMC article.
-
Rebound from Inhibition: Self-Correction against Neurodegeneration?J Clin Cell Immunol. 2017 Apr;8(2):492. doi: 10.4172/2155-9899.1000492. Epub 2017 Mar 13. J Clin Cell Immunol. 2017. PMID: 28775912 Free PMC article.
References
-
- Barber RP, Vaughn JE, Roberts E. The cytoarchitecture of GABAergic neurons in rat spinal cord. Brain Res. 1982;238:305–328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
