Rapid, learning-induced inhibitory synaptogenesis in murine barrel field
- PMID: 20089926
- PMCID: PMC2842932
- DOI: 10.1523/JNEUROSCI.2970-09.2010
Rapid, learning-induced inhibitory synaptogenesis in murine barrel field
Abstract
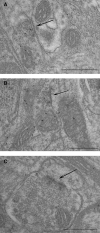
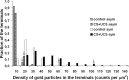
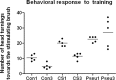
The structure of neurons changes during development and in response to injury or alteration in sensory experience. Changes occur in the number, shape, and dimensions of dendritic spines together with their synapses. However, precise data on these changes in response to learning are sparse. Here, we show using quantitative transmission electron microscopy that a simple form of learning involving mystacial vibrissae results in approximately 70% increase in the density of inhibitory synapses on spines of neurons located in layer IV barrels that represent the stimulated vibrissae. The spines contain one asymmetrical (excitatory) and one symmetrical (inhibitory) synapse (double-synapse spines), and their density increases threefold as a result of learning with no apparent change in the density of asymmetrical synapses. This effect seems to be specific for learning because pseudoconditioning (in which the conditioned and unconditioned stimuli are delivered at random) does not lead to the enhancement of symmetrical synapses but instead results in an upregulation of asymmetrical synapses on spines. Symmetrical synapses of cells located in barrels receiving the conditioned stimulus also show a greater concentration of GABA in their presynaptic terminals. These results indicate that the immediate effect of classical conditioning in the "conditioned" barrels is rapid, pronounced, and inhibitory.
Figures



References
-
- Armstrong-James M, Fox K. Spatiotemporal convergence and divergence in the rat S1 “barrel” cortex. J Comp Neurol. 1987;263:265–281. - PubMed
-
- Aroniadou-Anderjaska V, Keller A. Intrinsic inhibitory pathways in mouse barrel cortex. Neuroreport. 1996;7:2363–2368. - PubMed
-
- Bailey CH, Kandel ER. Synaptic remodeling, synaptic growth and the storage of long-term memory in Aplysia . Prog Brain Res. 2008;169:179–198. - PubMed
-
- Costa-Mattioli M, Sonenberg N. Translational control of gene expression: a molecular switch for memory storage. Prog Brain Res. 2008;169:81–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
