Regulation of gap junction coupling in bovine ciliary epithelium
- PMID: 20089928
- PMCID: PMC2853215
- DOI: 10.1152/ajpcell.00406.2009
Regulation of gap junction coupling in bovine ciliary epithelium
Abstract
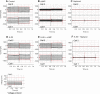
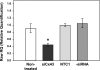
Aqueous humor is formed by fluid transfer from the ciliary stroma sequentially across the pigmented ciliary epithelial (PE) cells, gap junctions, and nonpigmented ciliary epithelial (NPE) cells. Which connexins (Cx) contribute to PE-NPE gap junctional formation appears species specific. We tested whether small interfering RNA (siRNA) against Cx43 (siCx43) affects bovine PE-NPE communication and whether cAMP affects communication. Native bovine ciliary epithelial cells were studied by dual-cell patch clamping, Lucifer Yellow (LY) transfer, quantitative polymerase chain reaction with reverse transcription (qRT-PCR), and Western immunoblot. qRT-PCR revealed at least 100-fold greater expression for Cx43 than Cx40. siCx43 knocked down target mRNA expression by 55 +/- 7% after 24 h, compared with nontargeting control siRNA (NTC1) transfection. After 48 h, siCx43 reduced Cx43 protein expression and LY transfer. The ratio of fluorescence intensity (R(f)) in recipient to donor cell was 0.47 +/- 0.09 (n = 11) 10 min after whole cell patch formation in couplets transfected with NTC1. siCx43 decreased R(f) by approximately 60% to 0.20 +/- 0.07 (n = 13, P < 0.02). Dibutyryl-cAMP (500 microM) also reduced LY dye transfer by approximately 60%, reducing R(f) from 0.41 +/- 0.05 (n = 15) to 0.17 +/- 0.05 (n = 20) after 10 min. Junctional currents were lowered by approximately 50% (n = 6) after 10-min perfusion with 500 microM dibutyryl-cAMP (n = 6); thereafter, heptanol abolished the currents (n = 5). Preincubation with the PKA inhibitor H-89 (2 microM) prevented cAMP-triggered current reduction (n = 6). We conclude that 1) Cx43, but not Cx40, is a major functional component of bovine PE-NPE gap junctions; and 2) under certain conditions, cAMP may act through PKA to inhibit bovine PE-NPE gap junctional communication.
Figures








Similar articles
-
Characterization and Regulation of Gap Junctions in Porcine Ciliary Epithelium.Invest Ophthalmol Vis Sci. 2018 Jul 2;59(8):3461-3468. doi: 10.1167/iovs.18-24682. Invest Ophthalmol Vis Sci. 2018. PMID: 30025101
-
Molecular profiling and cellular localization of connexin isoforms in the rat ciliary epithelium.Exp Eye Res. 2002 Jul;75(1):9-21. doi: 10.1006/exer.2002.1187. Exp Eye Res. 2002. PMID: 12123633
-
Electron microprobe analysis of ouabain-exposed ciliary epithelium: PE-NPE cell couplets form the functional units.Am J Physiol Cell Physiol. 2004 Jun;286(6):C1376-89. doi: 10.1152/ajpcell.00248.2003. Epub 2004 Feb 4. Am J Physiol Cell Physiol. 2004. PMID: 14761890
-
Basis of chloride transport in ciliary epithelium.J Membr Biol. 2004 Jul 1;200(1):1-13. doi: 10.1007/s00232-004-0688-5. J Membr Biol. 2004. PMID: 15386155 Review.
-
Cross-talk between pulmonary injury, oxidant stress, and gap junctional communication.Antioxid Redox Signal. 2009 Feb;11(2):355-67. doi: 10.1089/ars.2008.2183. Antioxid Redox Signal. 2009. PMID: 18816185 Free PMC article. Review.
Cited by
-
Terbutaline, forskolin and cAMP reduce secretion of aqueous humour in the isolated bovine eye.PLoS One. 2020 Dec 21;15(12):e0244253. doi: 10.1371/journal.pone.0244253. eCollection 2020. PLoS One. 2020. PMID: 33347508 Free PMC article.
-
The Significance of TRPV4 Channels and Hemichannels in the Lens and Ciliary Epithelium.J Ocul Pharmacol Ther. 2016 Oct;32(8):504-508. doi: 10.1089/jop.2016.0054. Epub 2016 Aug 11. J Ocul Pharmacol Ther. 2016. PMID: 27513167 Free PMC article. Review.
-
Pathways for ATP release by bovine ciliary epithelial cells, the initial step in purinergic regulation of aqueous humor inflow.Am J Physiol Cell Physiol. 2010 Dec;299(6):C1308-17. doi: 10.1152/ajpcell.00333.2010. Epub 2010 Oct 6. Am J Physiol Cell Physiol. 2010. PMID: 20926783 Free PMC article.
-
Mechanism of PKA-dependent and lipid-raft independent stimulation of Connexin43 expression by oxytoxin in mouse embryonic stem cells.Mol Endocrinol. 2012 Jul;26(7):1144-57. doi: 10.1210/me.2011-1343. Epub 2012 May 7. Mol Endocrinol. 2012. PMID: 22564436 Free PMC article.
-
Regulation of Aqueous Humor Secretion by Melatonin in Porcine Ciliary Epithelium.Int J Mol Sci. 2023 Mar 17;24(6):5789. doi: 10.3390/ijms24065789. Int J Mol Sci. 2023. PMID: 36982863 Free PMC article.
References
-
- Akaike N. Gramicidin perforated patch recording and intracellular chloride activity in excitable cells. Prog Biophys Mol Biol 65: 251–264, 1996 - PubMed
-
- Anguíta J, Chalfant ML, Civan MM, Coca-Prados M. Molecular cloning of the human volume-sensitive chloride conductance regulatory protein, pICln, from ocular ciliary epithelium. Biochem Biophys Res Commun 208: 89–95, 1995 - PubMed
-
- Brubaker RF. Clinical measurement of aqueous dynamics: implications for addressing glaucoma. In: Eye's Aqueous Humor: From Secretion to Glaucoma, edited by Civan MM. San Diego, CA: Academic, 1998, p. 234–284
-
- Caprioli J, Sears M, Bausher L, Gregory D, Mead A. Forskolin lowers intraocular pressure by reducing aqueous inflow. Invest Ophthalmol Vis Sci 25: 268–277, 1984 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

