Randomized trial of adjunctive topiramate therapy in infants with refractory partial seizures
- PMID: 20089937
- PMCID: PMC2836871
- DOI: 10.1212/WNL.0b013e3181d1cd4c
Randomized trial of adjunctive topiramate therapy in infants with refractory partial seizures
Abstract
Objective: To evaluate the efficacy and safety of adjunctive topiramate (sprinkle capsules or oral liquid) in reducing daily rates of partial-onset seizures (POS) in infants with refractory POS.
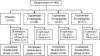
Methods: In this double-blind, placebo-controlled, parallel-group, international study, infants (n = 149) with clinical or EEG evidence of refractory POS were randomly allocated (1:1:1:1) to receive adjunctive topiramate 5, 15, or 25 mg/kg/d or placebo for 20 days. The primary variable was the median percentage reductions in daily POS rate from baseline to final assessment as recorded on a 48-hour video-EEG.
Results: Of the 149 infants (mean age 12 months) included in the intent-to-treat analysis set, 130 completed the study. Median percentage reduction from baseline in daily POS rate was not significantly different (p = 0.97) between topiramate 25 mg/kg (20.4%) and placebo (13.1%). Lower doses were not formally tested, but nominal p values for comparisons with placebo were not significant (15-mg/kg/d dose: p = 0.97; 5-mg/kg/d dose: p = 0.91). Treatment-emergent fever, diarrhea, vomiting, anorexia, weight decrease, somnolence, and viral infection occurred more frequently (> or = 10% difference) with topiramate than with placebo.
Conclusion: In infants aged 1-24 months, topiramate 5, 15, or 25 mg/kg/d was not effective as adjunctive treatment for refractory partial-onset seizures. No new safety concerns associated with topiramate use were noted.
Classification of evidence: This interventional study provides Class I evidence that topiramate 5, 15, or 25 mg/kg/d compared with placebo does not significantly reduce seizure rates in infants aged 1 month to 2 years with refractory partial-onset seizures.
Figures


Comment in
-
Epilepsy in infancy: answering the call.Neurology. 2010 Mar 2;74(9):708-9. doi: 10.1212/WNL.0b013e3181d1cd65. Epub 2010 Jan 27. Neurology. 2010. PMID: 20107140 No abstract available.
References
-
- Kramer U. Epilepsy in the first year of life: a review. J Child Neurol 1999;148:485–489. - PubMed
-
- Nordli DR. Diagnostic difficulty in infants and children. J Child Neurol 2002;17(suppl 1):S28–S35. - PubMed
-
- Korff CM, Nordli DR Jr. Epilepsy syndromes in infancy. Pediatr Neurol 2006;344:253–263. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
