Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2
- PMID: 20089951
- PMCID: PMC2838503
- DOI: 10.1056/NEJMoa0904849
Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2
Abstract
Background: Most persons who are infected with human immunodeficiency virus type 1 (HIV-1) are also infected with herpes simplex virus type 2 (HSV-2), which is frequently reactivated and is associated with increased plasma and genital levels of HIV-1. Therapy to suppress HSV-2 reduces the frequency of reactivation of HSV-2 as well as HIV-1 levels, suggesting that suppression of HSV-2 may reduce the risk of transmission of HIV-1.
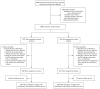
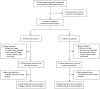
Methods: We conducted a randomized, placebo-controlled trial of suppressive therapy for HSV-2 (acyclovir at a dose of 400 mg orally twice daily) in couples in which only one of the partners was seropositive for HIV-1 (CD4 count, > or = 250 cells per cubic millimeter) and that partner was also infected with HSV-2 and was not taking antiretroviral therapy at the time of enrollment. The primary end point was transmission of HIV-1 to the partner who was not initially infected with HIV-1; linkage of transmissions was assessed by means of genetic sequencing of viruses.
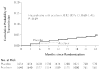
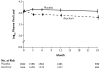
Results: A total of 3408 couples were enrolled at 14 sites in Africa. Of the partners who were infected with HIV-1, 68% were women, and the baseline median CD4 count was 462 cells per cubic millimeter. Of 132 HIV-1 seroconversions that occurred after randomization (an incidence of 2.7 per 100 person-years), 84 were linked within couples by viral sequencing: 41 in the acyclovir group and 43 in the placebo group (hazard ratio with acyclovir, 0.92, 95% confidence interval [CI], 0.60 to 1.41; P=0.69). Suppression with acyclovir reduced the mean plasma concentration of HIV-1 by 0.25 log(10) copies per milliliter (95% CI, 0.22 to 0.29; P<0.001) and the occurrence of HSV-2-positive genital ulcers by 73% (risk ratio, 0.27; 95% CI, 0.20 to 0.36; P<0.001). A total of 92% of the partners infected with HIV-1 and 84% of the partners not infected with HIV-1 remained in the study for 24 months. The level of adherence to the dispensed study drug was 96%. No serious adverse events related to acyclovir were observed.
Conclusions: Daily acyclovir therapy did not reduce the risk of transmission of HIV-1, despite a reduction in plasma HIV-1 RNA of 0.25 log(10) copies per milliliter and a 73% reduction in the occurrence of genital ulcers due to HSV-2. (ClinicalTrials.gov number, NCT00194519.)
2010 Massachusetts Medical Society
Figures

Comment in
-
Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2.N Engl J Med. 2010 May 6;362(18):1740; author reply 1741-2. doi: 10.1056/NEJMc1002408. N Engl J Med. 2010. PMID: 20445189 No abstract available.
-
Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2.N Engl J Med. 2010 May 6;362(18):1741; author reply 1741-2. N Engl J Med. 2010. PMID: 20449879 No abstract available.
References
-
- Weiss H. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes. 2004;11(Suppl 1):24A–35A. - PubMed
-
- Siegal FP, Lopez C, Hammer GS, et al. Severe acquired immunodeficiency in male homosexuals, manifested by chronic perianal ulcerative herpes simplex lesions. N Engl J Med. 1981;305:1439–44. - PubMed
-
- Strick LB, Wald A, Celum C. Management of herpes simplex virus type 2 infection in HIV type 1-infected persons. Clin Infect Dis. 2006;43:347–56. - PubMed
-
- Zuckerman RA, Lucchetti A, Whittington WL, et al. Herpes simplex virus (HSV) suppression with valacyclovir reduces rectal and blood plasma HIV-1 levels in HIV-1/HSV-2-seropositive men: a randomized, double-blind, placebo-controlled crossover trial. J Infect Dis. 2007;196:1500–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
