Altered levels of acetylcholinesterase in Alzheimer plasma
- PMID: 20090844
- PMCID: PMC2806824
- DOI: 10.1371/journal.pone.0008701
Altered levels of acetylcholinesterase in Alzheimer plasma
Abstract
Background: Many studies have been conducted in an extensive effort to identify alterations in blood cholinesterase levels as a consequence of disease, including the analysis of acetylcholinesterase (AChE) in plasma. Conventional assays using selective cholinesterase inhibitors have not been particularly successful as excess amounts of butyrylcholinesterase (BuChE) pose a major problem.
Principal findings: Here we have estimated the levels of AChE activity in human plasma by first immunoprecipitating BuChE and measuring AChE activity in the immunodepleted plasma. Human plasma AChE activity levels were approximately 20 nmol/min/mL, about 160 times lower than BuChE. The majority of AChE species are the light G(1)+G(2) forms and not G(4) tetramers. The levels and pattern of the molecular forms are similar to that observed in individuals with silent BuChE. We have also compared plasma AChE with the enzyme pattern obtained from human liver, red blood cells, cerebrospinal fluid (CSF) and brain, by sedimentation analysis, Western blotting and lectin-binding analysis. Finally, a selective increase of AChE activity was detected in plasma from Alzheimer's disease (AD) patients compared to age and gender-matched controls. This increase correlates with an increase in the G(1)+G(2) forms, the subset of AChE species which are increased in Alzheimer's brain. Western blot analysis demonstrated that a 78 kDa immunoreactive AChE protein band was also increased in Alzheimer's plasma, attributed in part to AChE-T subunits common in brain and CSF.
Conclusion: Plasma AChE might have potential as an indicator of disease progress and prognosis in AD and warrants further investigation.
Conflict of interest statement
Figures


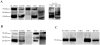


Similar articles
-
Inhibition of acetyl- and butyryl-cholinesterase in the cerebrospinal fluid of patients with Alzheimer's disease by rivastigmine: correlation with cognitive benefit.J Neural Transm (Vienna). 2002 Jul;109(7-8):1053-65. doi: 10.1007/s007020200089. J Neural Transm (Vienna). 2002. PMID: 12111443 Clinical Trial.
-
The recovery of cerebrospinal fluid acetylcholinesterase activity in Alzheimer's disease patients after treatment with metrifonate.Methods Find Exp Clin Pharmacol. 2000 Jan-Feb;22(1):57-61. doi: 10.1358/mf.2000.22.1.795849. Methods Find Exp Clin Pharmacol. 2000. PMID: 10791297 Clinical Trial.
-
Status of acetylcholinesterase and butyrylcholinesterase in Alzheimer's disease and type 2 diabetes mellitus.CNS Neurol Disord Drug Targets. 2014;13(8):1432-9. doi: 10.2174/1871527313666141023141545. CNS Neurol Disord Drug Targets. 2014. PMID: 25345511 Free PMC article. Review.
-
Acetylcholinesterase and butyrylcholinesterase activities in cerebrospinal fluid from different levels of the neuraxis of patients with dementia of the Alzheimer type.J Neurol Neurosurg Psychiatry. 1992 Nov;55(11):1074-8. doi: 10.1136/jnnp.55.11.1074. J Neurol Neurosurg Psychiatry. 1992. PMID: 1469405 Free PMC article.
-
Cholinesterases: new roles in brain function and in Alzheimer's disease.Neurochem Res. 2003 Apr;28(3-4):515-22. doi: 10.1023/a:1022869222652. Neurochem Res. 2003. PMID: 12675140 Review.
Cited by
-
Novel insights on acetylcholinesterase inhibition by Convolvulus pluricaulis, scopolamine and their combination in zebrafish.Nat Prod Bioprospect. 2022 Feb 25;12(1):6. doi: 10.1007/s13659-022-00332-5. Nat Prod Bioprospect. 2022. PMID: 35212831 Free PMC article.
-
Ameliorative role of Polyscias fruticosa leaf extract in aluminum chloride-induced neurotoxicity flies possibly mediated by N-methyl-D-aspartate receptor antagonistic and anticholinesterase active compounds.J Nat Med. 2025 Sep;79(5):1167-1187. doi: 10.1007/s11418-025-01928-0. Epub 2025 Jul 11. J Nat Med. 2025. PMID: 40646316
-
Honey and Alzheimer's Disease-Current Understanding and Future Prospects.Antioxidants (Basel). 2023 Feb 9;12(2):427. doi: 10.3390/antiox12020427. Antioxidants (Basel). 2023. PMID: 36829985 Free PMC article. Review.
-
Phenotypic Displays of Cholinergic Enzymes Associate With Markers of Inflammation, Neurofibrillary Tangles, and Neurodegeneration in Pre- and Early Symptomatic Dementia Subjects.Front Aging Neurosci. 2022 May 26;14:876019. doi: 10.3389/fnagi.2022.876019. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35693340 Free PMC article.
-
Alterations in Immune Responses Are Associated with Dysfunctional Intracellular Signaling in Peripheral Blood Mononuclear Cells of Men and Women with Mild Cognitive Impairment and Alzheimer's disease.Mol Neurobiol. 2024 May;61(5):2964-2977. doi: 10.1007/s12035-023-03764-3. Epub 2023 Nov 13. Mol Neurobiol. 2024. PMID: 37957423
References
-
- Davies P, Maloney AJF. Selective loss of central cholinergic neurons in Alzheimer's disease. Lancet. 1976;2:1403. - PubMed
-
- Perry EK, Perry RH, Blessed G, Tomlinson BE. Necropsy evidence of central cholinergic deficits in senile dementia. Lancet. 1977;1:189. - PubMed
-
- Mesulam MM, Morán MA. Cholinesterases within neurofibrillary tangles related to age and Alzheimer's disease. Ann Neurol. 1987;22:223–228. - PubMed
-
- Ulrich J, Meier-Ruge W, Probst A, Meier E, Ipsen S. Senile plaques: staining for acetylcholinesterase and A4 protein: a comparative study in the hippocampus and entorhinal cortex. Acta Neuropathol. 1990;80(6):624–628. - PubMed
-
- Inestrosa NC, Alvarez A, Perez CA, Moreno RD, Vicente M, et al. Acetylcholinesterase accelerates assembly of amyloid-beta-peptides into Alzheimer's fibrils: possible role of the peripheral site of the enzyme. Neuron. 1996;16:881–891. - PubMed

