Mice lacking thyroid hormone receptor Beta show enhanced apoptosis and delayed liver commitment for proliferation after partial hepatectomy
- PMID: 20090848
- PMCID: PMC2806828
- DOI: 10.1371/journal.pone.0008710
Mice lacking thyroid hormone receptor Beta show enhanced apoptosis and delayed liver commitment for proliferation after partial hepatectomy
Abstract
Background: The role of thyroid hormones and their receptors (TR) during liver regeneration after partial hepatectomy (PH) was studied using genetic and pharmacologic approaches. Roles in liver regeneration have been suggested for T3, but there is no clear evidence distinguishing the contribution of increased amounts of T3 from the modulation by unoccupied TRs.
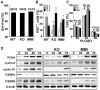
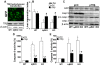
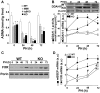
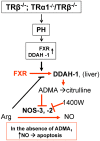
Methodology/principal findings: Mice lacking TRalpha1/TRbeta or TRbeta alone fully regenerated liver mass after PH, but showed delayed commitment to the initial round of hepatocyte proliferation and transient but intense apoptosis at 48h post-PH, affecting approximately 30% of the remaining hepatocytes. Pharmacologically induced hypothyroidism yielded similar results. Loss of TR activity was associated with enhanced nitrosative stress in the liver remnant, due to an increase in the activity of the nitric oxide synthase (NOS) 2 and 3, caused by a transient decrease in the concentration of asymmetric dimethylarginine (ADMA), a potent NOS inhibitor. This decrease in the ADMA levels was due to the presence of a higher activity of dimethylarginineaminohydrolase-1 (DDAH-1) in the regenerating liver of animals lacking TRalpha1/TRbeta or TRbeta. DDAH-1 expression and activity was paralleled by the activity of FXR, a transcription factor involved in liver regeneration and up-regulated in the absence of TR.
Conclusions/significance: We report that TRs are not required for liver regeneration; however, hypothyroid mice and TRbeta- or TRalpha1/TRbeta-deficient mice exhibit a delay in the restoration of liver mass, suggesting a specific role for TRbeta in liver regeneration. Altered regenerative responses are related with a delay in the expression of cyclins D1 and E, and the occurrence of liver apoptosis in the absence of activated TRbeta that can be prevented by administration of NOS inhibitors. Taken together, these results indicate that TRbeta contributes significantly to the rapid initial round of hepatocyte proliferation following PH, and improves the survival of the regenerating liver at later times.
Conflict of interest statement
Figures




Similar articles
-
Canonical Thyroid Hormone Receptor β Action Stimulates Hepatocyte Proliferation in Male Mice.Endocrinology. 2022 Mar 1;163(3):bqac003. doi: 10.1210/endocr/bqac003. Endocrinology. 2022. PMID: 35038735
-
Lacking thyroid hormone receptor beta gene does not influence alterations in peripheral thyroid hormone metabolism during acute illness.J Endocrinol. 2008 Apr;197(1):151-8. doi: 10.1677/JOE-07-0601. J Endocrinol. 2008. PMID: 18372241
-
An unliganded thyroid hormone beta receptor activates the cyclin D1/cyclin-dependent kinase/retinoblastoma/E2F pathway and induces pituitary tumorigenesis.Mol Cell Biol. 2005 Jan;25(1):124-35. doi: 10.1128/MCB.25.1.124-135.2005. Mol Cell Biol. 2005. PMID: 15601836 Free PMC article.
-
Signal transduction during liver regeneration.J Gastroenterol Hepatol. 1998 Sep;13 Suppl:S93-5. J Gastroenterol Hepatol. 1998. PMID: 9792040 Review.
-
Key hepatoprotective roles of mitochondria in liver regeneration.Am J Physiol Gastrointest Liver Physiol. 2023 Mar 1;324(3):G207-G218. doi: 10.1152/ajpgi.00220.2022. Epub 2023 Jan 17. Am J Physiol Gastrointest Liver Physiol. 2023. PMID: 36648139 Free PMC article. Review.
Cited by
-
Hormonal Contribution to Liver Regeneration.Mayo Clin Proc Innov Qual Outcomes. 2020 Jun 5;4(3):315-338. doi: 10.1016/j.mayocpiqo.2020.02.001. eCollection 2020 Jun. Mayo Clin Proc Innov Qual Outcomes. 2020. PMID: 32542223 Free PMC article. Review.
-
Clustering nuclear receptors in liver regeneration identifies candidate modulators of hepatocyte proliferation and hepatocarcinoma.PLoS One. 2014 Aug 12;9(8):e104449. doi: 10.1371/journal.pone.0104449. eCollection 2014. PLoS One. 2014. PMID: 25116592 Free PMC article.
-
A thyroid hormone receptor/KLF9 axis in human hepatocytes and pluripotent stem cells.Stem Cells. 2015 Feb;33(2):416-28. doi: 10.1002/stem.1875. Stem Cells. 2015. PMID: 25330987 Free PMC article.
-
Pregnancy and weaning regulate human maternal liver size and function.Proc Natl Acad Sci U S A. 2021 Nov 30;118(48):e2107269118. doi: 10.1073/pnas.2107269118. Proc Natl Acad Sci U S A. 2021. PMID: 34815335 Free PMC article.
-
Targeting Thyroid Hormone/Thyroid Hormone Receptor Axis: An Attractive Therapy Strategy in Liver Diseases.Front Pharmacol. 2022 Jun 2;13:871100. doi: 10.3389/fphar.2022.871100. eCollection 2022. Front Pharmacol. 2022. PMID: 35721201 Free PMC article. Review.
References
-
- Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. - PubMed
-
- Fausto N. Liver regeneration. JHepatol. 2000;32:19–31. - PubMed
-
- Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–53. - PubMed
-
- Alisi A, Demori I, Spagnuolo S, Pierantozzi E, Fugassa E, et al. Thyroid status affects rat liver regeneration after partial hepatectomy by regulating cell cycle and apoptosis. Cell Physiol Biochem. 2005;15:69–76. - PubMed
-
- Flores-Morales A, Gullberg H, Fernandez L, Stahlberg N, Lee NH, et al. Patterns of liver gene expression governed by TRbeta. Mol Endocrinol. 2002;16:1257–1268. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

