Synthesis of Boc-protected bicycloproline
- PMID: 20090865
- PMCID: PMC2808134
- DOI: 10.1016/j.tetlet.2009.03.092
Synthesis of Boc-protected bicycloproline
Abstract
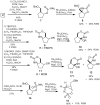
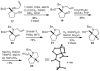
The synthesis of a highly constrained quaternary carbocyclic α-amino acid, (+)-N-Boc-bicycloproline, has been achieved starting from sodium cyclopentadienylide. Key steps include a rhodium-catalyzed nitrenoid C-H insertion to install the tert-alkylamine and a ring-closing metathesis reaction to form the pyrrolidine ring.
Figures

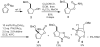

Similar articles
-
Conformationally constrained alpha-boc-aminophosphonates via transition metal-catalyzed/curtius rearrangement strategies.J Org Chem. 2002 Nov 15;67(23):8123-9. doi: 10.1021/jo0262208. J Org Chem. 2002. PMID: 12423141
-
Locked conformations for proline pyrrolidine ring: synthesis and conformational analysis of cis- and trans-4-tert-butylprolines.J Org Chem. 2005 Aug 5;70(16):6447-53. doi: 10.1021/jo050838a. J Org Chem. 2005. PMID: 16050708
-
Gold(I)-Catalyzed Ring-Closing Alkyne-Carbonyl Metathesis for the Synthesis of Butenolides.Chemistry. 2023 Nov 21;29(65):e202302044. doi: 10.1002/chem.202302044. Epub 2023 Oct 13. Chemistry. 2023. PMID: 37652895
-
Enantioselective Synthesis of cis- and trans-Cycloheptyl β-Fluoro Amines by Sequential aza-Henry Addition/Ring-Closing Metathesis.Org Lett. 2023 Feb 17;25(6):950-955. doi: 10.1021/acs.orglett.2c04285. Epub 2023 Feb 3. Org Lett. 2023. PMID: 36735762 Free PMC article.
-
Hydrogen-mediated reductive coupling of conjugated alkynes with ethyl (N-Sulfinyl)iminoacetates: synthesis of unnatural alpha-amino acids via rhodium-catalyzed C-C bond forming hydrogenation.J Am Chem Soc. 2005 Aug 17;127(32):11269-76. doi: 10.1021/ja051104i. J Am Chem Soc. 2005. PMID: 16089454 Free PMC article.
Cited by
-
Enantioselective synthesis of angularly substituted 1-azabicylic rings: coupled dynamic kinetic epimerization and chirality transfer.J Org Chem. 2013 Oct 4;78(19):9929-48. doi: 10.1021/jo4018099. Epub 2013 Sep 10. J Org Chem. 2013. PMID: 24090405 Free PMC article.
References
-
- Venkatraman J, Shankaramma SC, Balaram P. Chem Rev. 2001;101:3131–3152. - PubMed
- Toniolo C, Crisma M, Formaggio F, Peggion C. Biopolymers. 2001;60:396–419. - PubMed
- Hruby VJ. Acc Chem Res. 2001;34:389–397. - PubMed
- Gibson SE, Guillo N, Tozer MJ. Tetrahedron. 1999;55:585–615.
- Scheraga HA. Chem Rev. 1971;71:195. - PubMed
- Casanovas J, Jimenez AI, Cativiela C, Nussinov R, Aleman C. J Org Chem. 2008;73:644–651. - PubMed
- Casanovas J, Nussinov R, Aleman C. J Org Chem. 2008;73:4205–4211. - PMC - PubMed
- Goodman M, Shao H. Pure App Chem. 1996;68:1303–1308.
- Tanaka M. Chem Pharm Bull. 2007;55:349–358. - PubMed
- Hanessian S, McNaughtonSmith G, Lombart HG, Lubell WD. Tetrahedron. 1997;53:12789–12854.
- Karle IL. Biopolymers. 2001;60:351–365. - PubMed
-
- Whitmore L, Wallace BA. In: Handbook of Biologically Active Peptides. Kastin AJ, editor. Elsevier; Burlington: 2006. pp. 83–88.
- Mauger AB. J Nat Prod. 1996;59:1205–1211. - PubMed
- He HY, Janso JE, Yang HY, Bernan VS, Lin SL, Yu K. J Nat Prod. 2006;69:736–741. - PubMed
- Ohfune Y, Shinada T. Eur J Org Chem. 2005:5127–5143. - PubMed
- Fietzek PP, Kuhn K. Mol Cell Biochem. 1975;8:141–157. - PubMed
-
-
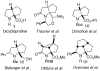
For reviews on the synthesis of quaternary α-amino acids see: Cativiela C, Diaz-de-Villegas MD. Tetrahedron: Asymmetry. 1998;9:3517–3599.Cativiela C, Diaz-de-Villegas MD. Tetrahedron: Asymmetry. 2000;11:645–732.Cativiela C, Diaz-de-Villegas MD. Tetrahedron: Asymmetry. 2007;18:569–623.Calaza MI, Cativiela C. Eur J Org Chem. 2008:3427–3448.Vogt H, Brase S. Org Biomol Chem. 2007;5:406–430.
-
-
- Dorsey AD, Barbarow JE, Trauner D. Org Lett. 2003;5:3237–3239. - PubMed
-
- Turner PG, Donohoe TJ, Cousins RPC. Chem Comm. 2004:1422–1423. - PubMed
- Belanger G, April M, Dauphin E, Roy S. J Org Chem. 2007;72:1104–1111. - PubMed
- Ohfune Y, Demura T, Iwama S, Matsuda H, Namba K, Shimamoto K, Shinada T. Tetrahedron Lett. 2003;44:5431–5434.
- Overman LE, Tellew JE. J Org Chem. 1996;61:8338–8340. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
