Pathogenic Bacterial Proteins and their Anti-Inflammatory Effects in the Eukaryotic Host
- PMID: 20090866
- PMCID: PMC2808140
- DOI: 10.2174/187152309789151986
Pathogenic Bacterial Proteins and their Anti-Inflammatory Effects in the Eukaryotic Host
Abstract
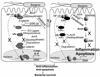
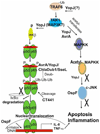
Bacteria use multiple strategies to bypass the inflammatory responses in order to survive in the host cells. In this review, we discuss the mechanism of the bacerial proteins in inhibiting inflammation. We highlight the anti-inflammatory roles of the type three secretion proteins including Salmonella AvrA, Enteropathogenic Escherichia coli Cif, and Yersinia YopJ, Staphylococcus aureus extracellular adherence protein, and Chlamydia proteins. We also discuss the research progress on the structures of these anti-inflammatory bacterial proteins. The current therapeutic methods for diseases, such as inflammatory bowel diseases, sclerosis, lack influence on the course of chronic inflammation and infection. Therefore, based on the molecular mechanism of the anti-inflammatory bacterial proteins and their 3-Dimension structure, we can design new peptides or non-peptidic molecules that serve as anti-inflammatory drugs without the possible side effect of promoting bacterial infection.
Figures
Similar articles
-
The Salmonella YopJ-homologue AvrA does not possess YopJ-like activity.Microb Pathog. 2000 Feb;28(2):59-70. doi: 10.1006/mpat.1999.0324. Microb Pathog. 2000. PMID: 10644492
-
Yersinia pseudotuberculosis YopJ Limits Macrophage Response by Downregulating COX-2-Mediated Biosynthesis of PGE2 in a MAPK/ERK-Dependent Manner.Microbiol Spectr. 2021 Sep 3;9(1):e0049621. doi: 10.1128/Spectrum.00496-21. Epub 2021 Jul 28. Microbiol Spectr. 2021. PMID: 34319170 Free PMC article.
-
The acetyltransferase activity of the bacterial toxin YopJ of Yersinia is activated by eukaryotic host cell inositol hexakisphosphate.J Biol Chem. 2010 Jun 25;285(26):19927-34. doi: 10.1074/jbc.M110.126581. Epub 2010 Apr 29. J Biol Chem. 2010. PMID: 20430892 Free PMC article.
-
Type III secretion: a bacterial device for close combat with cells of their eukaryotic host.Philos Trans R Soc Lond B Biol Sci. 2000 May 29;355(1397):681-93. doi: 10.1098/rstb.2000.0608. Philos Trans R Soc Lond B Biol Sci. 2000. PMID: 10874740 Free PMC article. Review.
-
The bacterial injection kit: type III secretion systems.Ann Med. 2005;37(4):234-49. doi: 10.1080/07853890510037329. Ann Med. 2005. PMID: 16019722 Review.
Cited by
-
AvrA effector protein of Salmonella enterica serovar Enteritidis is expressed and translocated in mesenteric lymph nodes at late stages of infection in mice.Microbiology (Reading). 2014 Jun;160(Pt 6):1191-1199. doi: 10.1099/mic.0.077115-0. Epub 2014 Apr 4. Microbiology (Reading). 2014. PMID: 24705228 Free PMC article.
-
Consistent activation of the β-catenin pathway by Salmonella type-three secretion effector protein AvrA in chronically infected intestine.Am J Physiol Gastrointest Liver Physiol. 2012 Nov 15;303(10):G1113-25. doi: 10.1152/ajpgi.00453.2011. Epub 2012 Sep 13. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 22982337 Free PMC article.
-
Protective Effects of High-Fat Diet against Murine Colitis in Association with Leptin Signaling and Gut Microbiome.Life (Basel). 2022 Jun 28;12(7):972. doi: 10.3390/life12070972. Life (Basel). 2022. PMID: 35888062 Free PMC article.
-
Presence of Salmonella AvrA in colorectal tumor and its precursor lesions in mouse intestine and human specimens.Oncotarget. 2017 Jul 6;8(33):55104-55115. doi: 10.18632/oncotarget.19052. eCollection 2017 Aug 15. Oncotarget. 2017. PMID: 28903406 Free PMC article.
-
Statewide Linkage Study of Salmonella Infection and Colorectal Cancer Incidence in Michigan, United States.J Registry Manag. 2024 Summer;51(2):62-68. J Registry Manag. 2024. PMID: 39184214 Free PMC article.
References
-
- Weiss U. Inflammation. Nature. 2008;454(7203):427. - PubMed
-
- Prat C, Bestebroer J, de Haas CJ, van Strijp JA, van Kessel KP. A new staphylococcal anti-inflammatory protein that antagonizes the formyl peptide receptor-like 1. J. Immunol. 2006;177(11):8017–8026. - PubMed
-
- Chavakis T, Hussain M, Kanse SM, Peters G, Bretzel RG, Flock JI, Herrmann M, Preissner KT. Staphylococcus aureus extracellular adherence protein serves as anti-inflammatory factor by inhibiting the recruitment of host leukocytes. Nat Med. 2002;8(7):687–693. - PubMed
-
- Peschel A. How do bacteria resist human antimicrobial peptides? Trends Microbiol. 2002;10(4):179–186. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
