Manipulating IL-2 availability amid presentation of donor MHC antigens suppresses murine alloimmune responses by inducing regulatory T cells
- PMID: 20090908
- PMCID: PMC2807454
- DOI: 10.1371/journal.pone.0008756
Manipulating IL-2 availability amid presentation of donor MHC antigens suppresses murine alloimmune responses by inducing regulatory T cells
Abstract
Background: Major histocompatibility complex (MHC) antigens are important for alloimmune responses as well as immune tolerance. Previous studies have shown that presentation of donor MHC antigens by donor-specific transfusion prior to or upon transplantation promotes transplant tolerance induced by other agents. However, it is unclear whether presentation of donor MHC antigens by DNA vaccination induces long-term allograft survival.
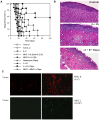
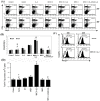
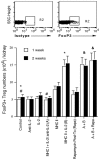
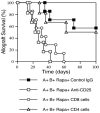
Methodology/principal findings: We investigated whether presentation of MHC class-II and/or class-I donor antigens by DNA vaccination suppresses alloimmune responses and promotes long-term allograft acceptance. We initially found that presentation of both MHC donor antigens by DNA vaccination itself prior to transplantation fails to significantly prolong islet allograft survival in otherwise untreated mice. However, islet allograft survival was significantly prolonged when MHC class-II DNA vaccination was accompanied with IL-2 administration (MHCII + IL-2) while MHC class-I DNA vaccination was followed by IL-2 and subsequent neutralizing anti-IL-2 treatments (MHCI + IL-2/anti-IL-2). Especially, this protocol promoted long-term allograft survival in the majority of recipients (57%) when combined with low doses of rapamycin post-transplantation. Importantly, MHCII + IL-2 induced FoxP3+ Treg cells in both spleens and grafts and suppressed graft-infiltrating CD4+ cell proliferation, whereas MHCI + IL-2/anti-IL-2 mainly inhibited graft-infiltrating CD8+ cell proliferation and donor-specific CTL activity. The combined protocol plus rapamycin treatment further reduced both CD4+ and CD8+ T cell proliferation as well as donor-specific CTL activity but spared FoxP3+ Treg cells. Depleting CD25+ Treg cells or adoptive transfer of pre-sensitized CD8+ T cells abolished this long-term allograft survival.
Conclusions/significance: Manipulating IL-2 availability during presentation of MHC class-II and class-I donor antigens by DNA vaccination pre-transplantation induces Treg cells, suppresses alloimmune responses and promotes long-term allograft survival.
Conflict of interest statement
Figures
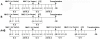


Similar articles
-
The role of Foxp3+ regulatory T cells in liver transplant tolerance.Transplant Proc. 2006 Dec;38(10):3205-6. doi: 10.1016/j.transproceed.2006.10.093. Transplant Proc. 2006. PMID: 17175223
-
Donor-antigen Inoculation in the Testis Promotes Skin Allograft Acceptance Induced by Conventional Costimulatory Blockade via Induction of CD8 + CD122+ and CD4 + CD25+ Regulatory T Cells.Transplantation. 2016 Apr;100(4):763-71. doi: 10.1097/TP.0000000000001011. Transplantation. 2016. PMID: 26569069
-
Alloantigen gene transfer to hepatocytes promotes tolerance to pancreatic islet graft by inducing CD8+ regulatory T cells.J Hepatol. 2017 Apr;66(4):765-777. doi: 10.1016/j.jhep.2016.11.019. Epub 2016 Nov 30. J Hepatol. 2017. PMID: 27914923
-
Presentation of donor major histocompatibility complex class II antigens by dna vaccination prolongs heart allograft survival.Transplantation. 2004 Mar 15;77(5):733-40. doi: 10.1097/01.tp.0000114613.70156.db. Transplantation. 2004. PMID: 15021837
-
The potential role of infusions of T regulatory cells in inducing and maintaining liver allograft tolerance.Clin Liver Dis (Hoboken). 2023 Jun 19;22(4):146-151. doi: 10.1097/CLD.0000000000000038. eCollection 2023 Oct. Clin Liver Dis (Hoboken). 2023. PMID: 37908871 Free PMC article. Review.
References
-
- Medawar PB. The use of antigenic tissue extracts to weaken the immunological reaction against skin homografts in mice. Transplantation. 1963;1:21–38. - PubMed
-
- Soulillou JP, Blandin F, Gunther E, Lemoine V. Genetics of the blood transfusion effect on heart allografts in rats. Transplantation. 1984;38:63–67. - PubMed
-
- Sayegh MH, Perico N, Imberti O, Hancock WW, Carpenter CB, et al. Thymic recognition of class II major histocompatibility complex allopeptides induces donor-specific unresponsiveness to renal allografts. Transplantation. 1993;56:461–465. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

